Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Dual specificity mitogen-activated protein kinase kinase 1
Ligand
BDBM50194042
Substrate
n/a
Meas. Tech.
ChEMBL_420098 (CHEMBL873740)
EC50
118±n/a nM
Citation
 El Abdellaoui, H; Varaprasad, CV; Barawkar, D; Chakravarty, S; Maderna, A; Tam, R; Chen, H; Allan, M; Wu, JZ; Appleby, T; Yan, S; Zhang, W; Lang, S; Yao, N; Hamatake, R; Hong, Z Identification of isothiazole-4-carboxamidines derivatives as a novel class of allosteric MEK1 inhibitors. Bioorg Med Chem Lett 16:5561-6 (2006) [PubMed] Article
El Abdellaoui, H; Varaprasad, CV; Barawkar, D; Chakravarty, S; Maderna, A; Tam, R; Chen, H; Allan, M; Wu, JZ; Appleby, T; Yan, S; Zhang, W; Lang, S; Yao, N; Hamatake, R; Hong, Z Identification of isothiazole-4-carboxamidines derivatives as a novel class of allosteric MEK1 inhibitors. Bioorg Med Chem Lett 16:5561-6 (2006) [PubMed] Article More Info.:
Target
Name:
Dual specificity mitogen-activated protein kinase kinase 1
Synonyms:
Dual specificity mitogen-activated protein kinase (MEK) | Dual specificity mitogen-activated protein kinase kinase 1 (MEK) | Dual specificity mitogen-activated protein kinase kinase 1 (MEK1) | Dual specificity mitogen-activated protein kinase kinase 1/Mitogen-activated protein kinase 1/RAF proto-oncogene serine/threonine-protein kinase | Dual specificity mitogen-activated protein kinase kinase MEK1/2 | ERK activator kinase 1 | MAP kinase kinase 1 | MAP2K1 | MAPK/ERK kinase 1 | MAPK/ERK kinase 1 (MEK1) | MEK-1 | MEK1 | MP2K1_HUMAN | Mitogen-activated protein kinase 1 (MEK1) | PRKMK1 | VHL-MAP2K1/MAP2K2
Type:
Other Protein Type
Mol. Mass.:
43439.03
Organism:
Homo sapiens (Human)
Description:
Full-length human MEK-1 was generated by PCR and purified as a fusion protein from Escherichia coli lysates.
Residue:
393
Sequence:
MPKKKPTPIQLNPAPDGSAVNGTSSAETNLEALQKKLEELELDEQQRKRLEAFLTQKQKVGELKDDDFEKISELGAGNGGVVFKVSHKPSGLVMARKLIHLEIKPAIRNQIIRELQVLHECNSPYIVGFYGAFYSDGEISICMEHMDGGSLDQVLKKAGRIPEQILGKVSIAVIKGLTYLREKHKIMHRDVKPSNILVNSRGEIKLCDFGVSGQLIDSMANSFVGTRSYMSPERLQGTHYSVQSDIWSMGLSLVEMAVGRYPIPPPDAKELELMFGCQVEGDAAETPPRPRTPGRPLSSYGMDSRPPMAIFELLDYIVNEPPPKLPSGVFSLEFQDFVNKCLIKNPAERADLKQLMVHAFIKRSDAEEVDFAGWLCSTIGLNQPSTPTHAAGV
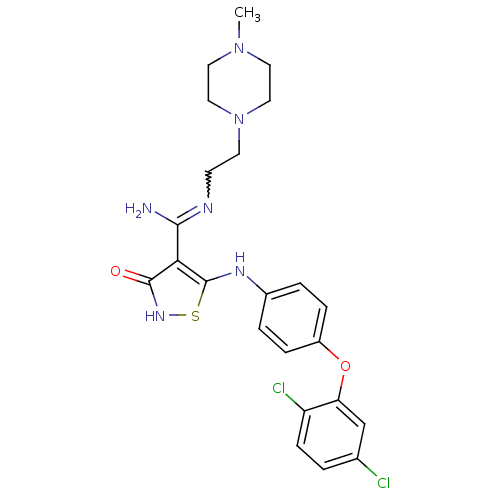
Inhibitor
Name:
BDBM50194042
Synonyms:
5-4-2,5-dichlorophenoxy)phenylamino)-3-hydroxy-N-2-4-methylpiperazin-1-yl)ethyl)isothiazole-4-carboximidamide | CHEMBL214977
Type:
Small organic molecule
Emp. Form.:
C23H26Cl2N6O2S
Mol. Mass.:
521.463
SMILES:
CN1CCN(CCN=C(N)c2c(Nc3ccc(Oc4cc(Cl)ccc4Cl)cc3)s[nH]c2=O)CC1 |w:7.6|