Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Endothelin receptor type B
Ligand
BDBM50286614
Substrate
n/a
Meas. Tech.
ChEMBL_63523 (CHEMBL677271)
IC50
1.2±n/a nM
Citation
 He, JX; Cody, WL; Flynn, MA; Welch, KM; Reynolds, EE; Doherty, AM Res-701-1, synthesis and a reevaluation of its effects on the endothelin receptors Bioorg Med Chem Lett 5:621-626 (1995) Article
He, JX; Cody, WL; Flynn, MA; Welch, KM; Reynolds, EE; Doherty, AM Res-701-1, synthesis and a reevaluation of its effects on the endothelin receptors Bioorg Med Chem Lett 5:621-626 (1995) Article More Info.:
Target
Name:
Endothelin receptor type B
Synonyms:
EDNRB | EDNRB_HUMAN | ENDOTHELIN B | ET-B | ETRB | Endothelin receptor ET-B | Endothelin receptor non-selective type | Endothelin receptor, ET-A/ET-B
Type:
Enzyme Catalytic Domain
Mol. Mass.:
49664.00
Organism:
Homo sapiens (Human)
Description:
ENDOTHELIN B EDNRB HUMAN::P24530
Residue:
442
Sequence:
MQPPPSLCGRALVALVLACGLSRIWGEERGFPPDRATPLLQTAEIMTPPTKTLWPKGSNASLARSLAPAEVPKGDRTAGSPPRTISPPPCQGPIEIKETFKYINTVVSCLVFVLGIIGNSTLLRIIYKNKCMRNGPNILIASLALGDLLHIVIDIPINVYKLLAEDWPFGAEMCKLVPFIQKASVGITVLSLCALSIDRYRAVASWSRIKGIGVPKWTAVEIVLIWVVSVVLAVPEAIGFDIITMDYKGSYLRICLLHPVQKTAFMQFYKTAKDWWLFSFYFCLPLAITAFFYTLMTCEMLRKKSGMQIALNDHLKQRREVAKTVFCLVLVFALCWLPLHLSRILKLTLYNQNDPNRCELLSFLLVLDYIGINMASLNSCINPIALYLVSKRFKNCFKSCLCCWCQSFEEKQSLEEKQSCLKFKANDHGYDNFRSSNKYSSS
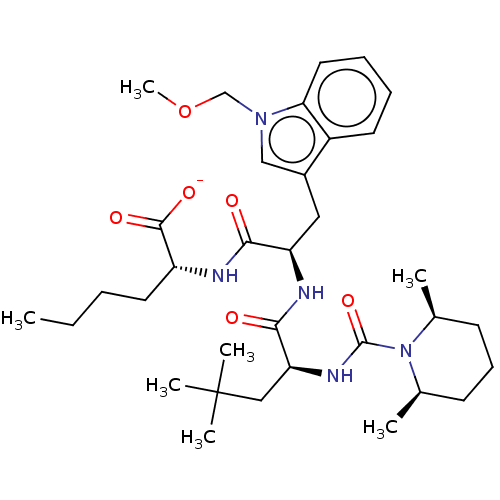
Inhibitor
Name:
BDBM50286614
Synonyms:
CHEMBL155943 | CHEMBL3143473 | Sodium; 2-[2-{2-[(2,6-dimethyl-piperidine-1-carbonyl)-amino]-4,4-dimethyl-pentanoylamino}-3-(1-methoxymethyl-1H-indol-3-yl)-propionylamino]-hexanoate
Type:
Small organic molecule
Emp. Form.:
C34H52N5NaO6
Mol. Mass.:
649.7963
SMILES:
[Na+].CCCC[C@@H](NC(=O)[C@@H](Cc1cn(COC)c2ccccc12)NC(=O)[C@H](CC(C)(C)C)NC(=O)N1[C@@H](C)CCC[C@H]1C)C([O-])=O |r|