Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
RAC-alpha serine/threonine-protein kinase
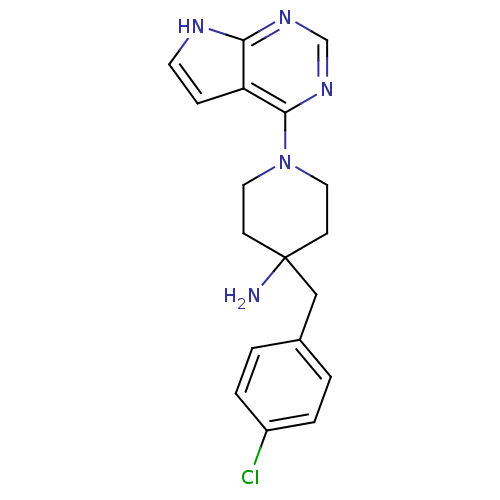
Ligand
BDBM50237622
Substrate
n/a
Meas. Tech.
ChEMBL_608354 (CHEMBL1074388)
IC50
0.66±n/a nM
Citation
 McHardy, T; Caldwell, JJ; Cheung, KM; Hunter, LJ; Taylor, K; Rowlands, M; Ruddle, R; Henley, A; de Haven Brandon, A; Valenti, M; Davies, TG; Fazal, L; Seavers, L; Raynaud, FI; Eccles, SA; Aherne, GW; Garrett, MD; Collins, I Discovery of 4-amino-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamides as selective, orally active inhibitors of protein kinase B (Akt). J Med Chem 53:2239-49 (2010) [PubMed] Article
McHardy, T; Caldwell, JJ; Cheung, KM; Hunter, LJ; Taylor, K; Rowlands, M; Ruddle, R; Henley, A; de Haven Brandon, A; Valenti, M; Davies, TG; Fazal, L; Seavers, L; Raynaud, FI; Eccles, SA; Aherne, GW; Garrett, MD; Collins, I Discovery of 4-amino-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamides as selective, orally active inhibitors of protein kinase B (Akt). J Med Chem 53:2239-49 (2010) [PubMed] Article More Info.:
Target
Name:
RAC-alpha serine/threonine-protein kinase
Synonyms:
AKT phosphorylation (p-AKT) | AKT1 | AKT1/PPP1CA | AKT1_HUMAN | C-AKT | PKB | PKB alpha | Protein kinase Akt-1 | Protein kinase B | Protein kinase B (AKT1) | Protein kinase B (Akt 1) | Protein kinase B (Akt) | Protein kinase B alpha | Protein kinase B alpha (AKT1) | Proto-oncogene Akt (Akt1) | Proto-oncogene c-Akt (AKT) | Proto-oncogene c-Akt (AKT1) | RAC | RAC-PK-alpha | RAC-alpha serine/threonine-protein kinase (AKT) | RAC-alpha serine/threonine-protein kinase (AKT1) | RAC-alpha serine/threonine-protein kinase (pAKT)
Type:
Enzyme
Mol. Mass.:
55681.25
Organism:
Homo sapiens (Human)
Description:
P31749
Residue:
480
Sequence:
MSDVAIVKEGWLHKRGEYIKTWRPRYFLLKNDGTFIGYKERPQDVDQREAPLNNFSVAQCQLMKTERPRPNTFIIRCLQWTTVIERTFHVETPEEREEWTTAIQTVADGLKKQEEEEMDFRSGSPSDNSGAEEMEVSLAKPKHRVTMNEFEYLKLLGKGTFGKVILVKEKATGRYYAMKILKKEVIVAKDEVAHTLTENRVLQNSRHPFLTALKYSFQTHDRLCFVMEYANGGELFFHLSRERVFSEDRARFYGAEIVSALDYLHSEKNVVYRDLKLENLMLDKDGHIKITDFGLCKEGIKDGATMKTFCGTPEYLAPEVLEDNDYGRAVDWWGLGVVMYEMMCGRLPFYNQDHEKLFELILMEEIRFPRTLGPEAKSLLSGLLKKDPKQRLGGGSEDAKEIMQHRFFAGIVWQHVYEKKLSPPFKPQVTSETDTRYFDEEFTAQMITITPPDQDDSMECVDSERRPHFPQFSYSASGTA
Inhibitor
Name:
BDBM50237622
Synonyms:
4-(4-Chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin- | 4-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-4-amine | CCT128930 | CHEMBL263664 | US8796293, 17
Type:
Small organic molecule
Emp. Form.:
C18H20ClN5
Mol. Mass.:
341.838
SMILES:
NC1(Cc2ccc(Cl)cc2)CCN(CC1)c1ncnc2[nH]ccc12