Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Inositol 1,4,5-trisphosphate receptor type 1
Ligand
BDBM50184325
Substrate
n/a
Meas. Tech.
ChEMBL_645182 (CHEMBL1218398)
EC50
1.5±n/a nM
Citation
 Rossi, AM; Riley, AM; Tovey, SC; Rahman, T; Dellis, O; Taylor, EJ; Veresov, VG; Potter, BV; Taylor, CW Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat Chem Biol 5:631-9 (2009) [PubMed] Article
Rossi, AM; Riley, AM; Tovey, SC; Rahman, T; Dellis, O; Taylor, EJ; Veresov, VG; Potter, BV; Taylor, CW Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat Chem Biol 5:631-9 (2009) [PubMed] Article More Info.:
Target
Name:
Inositol 1,4,5-trisphosphate receptor type 1
Synonyms:
ITPR1_RAT | Insp3r | Itpr1
Type:
PROTEIN
Mol. Mass.:
313234.63
Organism:
Rattus norvegicus
Description:
ChEMBL_818416
Residue:
2750
Sequence:
MSDKMSSFLHIGDICSLYAEGSTNGFISTLGLVDDRCVVQPEAGDLNNPPKKFRDCLFKLCPMNRYSAQKQFWKAAKPGANSTTDAVLLNKLHHAADLEKKQNETENRKLLGTVIQYGNVIQLLHLKSNKYLTVNKRLPALLEKNAMRVTLDEAGNEGSWFYIQPFYKLRSIGDSVVIGDKVVLNPVNAGQPLHASSHQLVDNPGCNEVNSVNCNTSWKIVLFMKWSDNKDDILKGGDVVRLFHAEQEKFLTCDEHRKKQHVFLRTTGRQSATSATSSKALWEVEVVQHDPCRGGAGYWNSLFRFKHLATGHYLAAEVDPDFEEECLEFQPSVDPDQDASRSRLRNAQEKMVYSLVSVPEGNDISSIFELDPTTLRGGDSLVPRNSYVRLRHLCTNTWVHSTNIPIDKEEEKPVMLKIGTSPLKEDKEAFAIVPVSPAEVRDLDFANDASKVLGSIAGKLEKGTITQNERRSVTKLLEDLVYFVTGGTNSGQDVLEVVFSKPNRERQKLMREQNILKQIFKLLQAPFTDCGDGPMLRLEELGDQRHAPFRHICRLCYRVLRHSQQDYRKNQEYIAKQFGFMQKQIGYDVLAEDTITALLHNNRKLLEKHITAAEIDTFVSLVRKNREPRFLDYLSDLCVSMNKSIPVTQELICKAVLNPTNADILIETKLVLSRFEFEGVSTGENALEAGEDEEEVWLFWRDSNKEIRSKSVRELAQDAKEGQKEDRDVLSYYRYQLNLFARMCLDRQYLAINEISGQLDVDLILRCMSDENLPYDLRASFCRLMLHMHVDRDPQEQVTPVKYARLWSEIPSEIAIDDYDSSGASKDEIKERFAQTMEFVEEYLRDVVCQRFPFSDKEKNKLTFEVVNLARNLIYFGFYNFSDLLRLTKILLAILDCVHVTTIFPISKMTKGEENKGSNVMRSIHGVGELMTQVVLRGGGFLPMTPMAAAPEGNVKQAEPEKEDIMVMDTKLKIIEILQFILNVRLDYRISCLLCIFKREFDESNSQSSETSSGNSSQEGPSNVPGALDFEHIEEQAEGIFGGSEENTPLDLDDHGGRTFLRVLLHLTMHDYPPLVSGALQLLFRHFSQRQEVLQAFKQVQLLVTSQDVDNYKQIKQDLDQLRSIVEKSELWVYKGQGPDEPMDGASGENEHKKTEEGTSKPLKHESTSSYNYRVVKEILIRLSKLCVQESASVRKSRKQQQRLLRNMGAHAVVLELLQIPYEKAEDTKMQEIMRLAHEFLQNFCAGNQQNQALLHKHINLFLNPGILEAVTMQHIFMNNFQLCSEINERVVQHFVHCIETHGRNVQYIKFLQTIVKAEGKFIKKCQDMVMAELVNSGEDVLVFYNDRASFQTLIQMMRSERDRMDENSPLFMYHIHLVELLAVCTEGKNVYTEIKCNSLLPLDDIVRVVTHEDCIPEVKIAYINFLNHCYVDTEVEMKEIYTSNHMWKLFENFLVDICRACNNTSDRKHADSVLEKYVTEIVMSIVTTFFSSPFSDQSTTLQTRQPVFVQLLQGVFRVYHCNWLMPSQKASVESCIRVLSDVAKSRAIAIPVDLDSQVNNLFLKSHNIVQKTAMNWRLSARNAARRDSVLAASRDYRNIIERLQDIVSALEDRLRPLVQAELSVLVDVLHRPELLFPENTDARRKCESGGFICKLIKHTKQLLEENEEKLCIKVLQTLREMMTKDRGYGEKQISIDELENAELPQPPEAENSTEQELEPSPPLRQLEDHKRGEALRQILVNRYYGNIRPSGRRESLTSFGNGPLSPGGPSKPGGGGGGPGSGSTSRGEMSLAEVQCHLDKEGASNLVIDLIMNASSDRVFHESILLAIALLEGGNTTIQHSFFCRLTEDKKSEKFFKVFYDRMKVAQQEIKATVTVNTSDLGNKKKDDEVDRDAPSRKKAKEPTTQITEEVRDQLLEASAATRKAFTTFRREADPDDHYQSGEGTQATTDKAKDDLEMSAVITIMQPILRFLQLLCENHNRDLQNFLRCQNNKTNYNLVCETLQFLDCICGSTTGGLGLLGLYINEKNVALINQTLESLTEYCQGPCHENQNCIATHESNGIDIITALILNDINPLGKKRMDLVLELKNNASKLLLAIMESRHDSENAERILYNMRPKELVEVIKKAYMQGEVEFEDGENGEDGAASPRNVGHNIYILAHQLARHNKELQTMLKPGGQVDGDEALEFYAKHTAQIEIVRLDRTMEQIVFPVPSICEFLTKESKLRIYYTTERDEQGSKINDFFLRSEDLFNEMNWQKKLRAQPVLYWCARNMSFWSSISFNLAVLMNLLVAFFYPFKGVRGGTLEPHWSGLLWTAMLISLAIVIALPKPHGIRALIASTILRLIFSVGLQPTLFLLGAFNVCNKIIFLMSFVGNCGTFTRGYRAMVLDVEFLYHLLYLLICAMGLFVHEFFYSLLLFDLVYREETLLNVIKSVTRNGRPIILTAALALILVYLFSIVGYLFFKDDFILEVDRLPNETAGPETGESLANDFLYSDVCRVETGENCTSPAPKEELLPVEETEQDKEHTCETLLMCIVTVLSHGLRSGGGVGDVLRKPSKEEPLFAARVIYDLLFFFMVIIIVLNLIFGVIIDTFADLRSEKQKKEEILKTTCFICGLERDKFDNKTVTFEEHIKEEHNMWHYLCFIVLVKVKDSTEYTGPESYVAEMIRERNLDWFPRMRAMSLVSSDSEGEQNELRNLQEKLESTMKLVTNLSGQLSELKDQMTEQRKQKQRIGLLGHPPHMNVNPQQPA
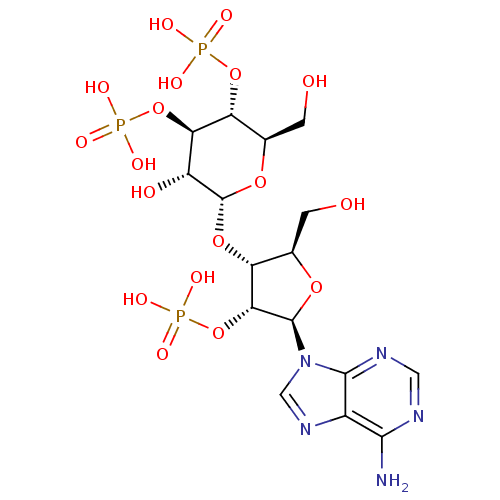
Inhibitor
Name:
BDBM50184325
Synonyms:
2-[5-(6-amino-9H-9-purinyl)-2-hydroxymethyl-4-oxyphosphate-(2R,3R,4R,5R)-tetrahydro-3-furanyloxy]-6-hydroxymethyl-4,5-dioxyphosphate-(2R,3R,4S,5R,6R)-tetrahydro-2H-3-pyranol | CHEMBL204385 | adenophostin A
Type:
Small organic molecule
Emp. Form.:
C16H26N5O18P3
Mol. Mass.:
669.3216
SMILES:
Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O[C@H]2O[C@H](CO)[C@@H](OP(O)(O)=O)[C@H](OP(O)(O)=O)[C@H]2O)[C@H]1OP(O)(O)=O