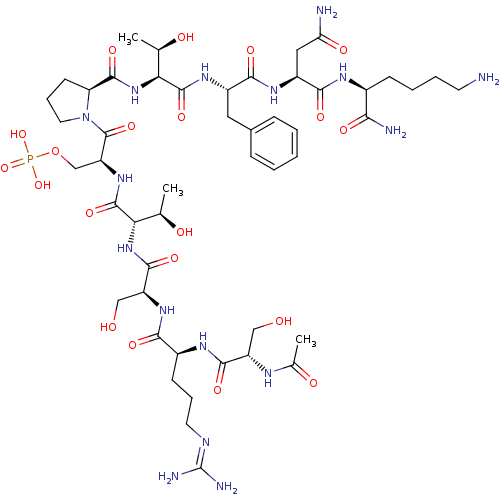
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Breast cancer type 1 susceptibility protein
Ligand
BDBM50345642
Substrate
n/a
Meas. Tech.
ChEMBL_752253 (CHEMBL1785942)
Kd
4.4E2±n/a nM
Citation
 Yuan, Z; Kumar, EA; Kizhake, S; Natarajan, A Structure-activity relationship studies to probe the phosphoprotein binding site on the carboxy terminal domains of the breast cancer susceptibility gene 1. J Med Chem 54:4264-8 (2011) [PubMed] Article
Yuan, Z; Kumar, EA; Kizhake, S; Natarajan, A Structure-activity relationship studies to probe the phosphoprotein binding site on the carboxy terminal domains of the breast cancer susceptibility gene 1. J Med Chem 54:4264-8 (2011) [PubMed] Article More Info.:
Target
Name:
Breast cancer type 1 susceptibility protein
Synonyms:
BRCA1 | BRCA1_HUMAN | RNF53
Type:
PROTEIN
Mol. Mass.:
207672.68
Organism:
Homo sapiens (Human)
Description:
ChEMBL_812733
Residue:
1863
Sequence:
MDLSALRVEEVQNVINAMQKILECPICLELIKEPVSTKCDHIFCKFCMLKLLNQKKGPSQCPLCKNDITKRSLQESTRFSQLVEELLKIICAFQLDTGLEYANSYNFAKKENNSPEHLKDEVSIIQSMGYRNRAKRLLQSEPENPSLQETSLSVQLSNLGTVRTLRTKQRIQPQKTSVYIELGSDSSEDTVNKATYCSVGDQELLQITPQGTRDEISLDSAKKAACEFSETDVTNTEHHQPSNNDLNTTEKRAAERHPEKYQGSSVSNLHVEPCGTNTHASSLQHENSSLLLTKDRMNVEKAEFCNKSKQPGLARSQHNRWAGSKETCNDRRTPSTEKKVDLNADPLCERKEWNKQKLPCSENPRDTEDVPWITLNSSIQKVNEWFSRSDELLGSDDSHDGESESNAKVADVLDVLNEVDEYSGSSEKIDLLASDPHEALICKSERVHSKSVESNIEDKIFGKTYRKKASLPNLSHVTENLIIGAFVTEPQIIQERPLTNKLKRKRRPTSGLHPEDFIKKADLAVQKTPEMINQGTNQTEQNGQVMNITNSGHENKTKGDSIQNEKNPNPIESLEKESAFKTKAEPISSSISNMELELNIHNSKAPKKNRLRRKSSTRHIHALELVVSRNLSPPNCTELQIDSCSSSEEIKKKKYNQMPVRHSRNLQLMEGKEPATGAKKSNKPNEQTSKRHDSDTFPELKLTNAPGSFTKCSNTSELKEFVNPSLPREEKEEKLETVKVSNNAEDPKDLMLSGERVLQTERSVESSSISLVPGTDYGTQESISLLEVSTLGKAKTEPNKCVSQCAAFENPKGLIHGCSKDNRNDTEGFKYPLGHEVNHSRETSIEMEESELDAQYLQNTFKVSKRQSFAPFSNPGNAEEECATFSAHSGSLKKQSPKVTFECEQKEENQGKNESNIKPVQTVNITAGFPVVGQKDKPVDNAKCSIKGGSRFCLSSQFRGNETGLITPNKHGLLQNPYRIPPLFPIKSFVKTKCKKNLLEENFEEHSMSPEREMGNENIPSTVSTISRNNIRENVFKEASSSNINEVGSSTNEVGSSINEIGSSDENIQAELGRNRGPKLNAMLRLGVLQPEVYKQSLPGSNCKHPEIKKQEYEEVVQTVNTDFSPYLISDNLEQPMGSSHASQVCSETPDDLLDDGEIKEDTSFAENDIKESSAVFSKSVQKGELSRSPSPFTHTHLAQGYRRGAKKLESSEENLSSEDEELPCFQHLLFGKVNNIPSQSTRHSTVATECLSKNTEENLLSLKNSLNDCSNQVILAKASQEHHLSEETKCSASLFSSQCSELEDLTANTNTQDPFLIGSSKQMRHQSESQGVGLSDKELVSDDEERGTGLEENNQEEQSMDSNLGEAASGCESETSVSEDCSGLSSQSDILTTQQRDTMQHNLIKLQQEMAELEAVLEQHGSQPSNSYPSIISDSSALEDLRNPEQSTSEKAVLTSQKSSEYPISQNPEGLSADKFEVSADSSTSKNKEPGVERSSPSKCPSLDDRWYMHSCSGSLQNRNYPSQEELIKVVDVEEQQLEESGPHDLTETSYLPRQDLEGTPYLESGISLFSDDPESDPSEDRAPESARVGNIPSSTSALKVPQLKVAESAQSPAAAHTTDTAGYNAMEESVSREKPELTASTERVNKRMSMVVSGLTPEEFMLVYKFARKHHITLTNLITEETTHVVMKTDAEFVCERTLKYFLGIAGGKWVVSYFWVTQSIKERKMLNEHDFEVRGDVVNGRNHQGPKRARESQDRKIFRGLEICCYGPFTNMPTDQLEWMVQLCGASVVKELSSFTLGTGVHPIVVVQPDAWTEDNGFHAIGQMCEAPVVTREWVLDSVALYQCQELDTYLIPQIPHSHY
Inhibitor
Name:
BDBM50345642
Synonyms:
(2S,5S,8S,11S,14S)-2-((S)-2-((2S,3R)-1-((S)-1-((S)-4-amino-1-((S)-1,6-diamino-1-oxohexan-2-ylamino)-1,4-dioxobutan-2-ylamino)-1-oxo-3-phenylpropan-2-ylamino)-3-hydroxy-1-oxobutan-2-ylcarbamoyl)pyrrolidine-1-carbonyl)-11-(3-guanidinopropyl)-5-((R)-1-hydroxyethyl)-8,14-bis(hydroxymethyl)-4,7,10,13,16-pentaoxo-3,6,9,12,15-pentaazaheptadecyl dihydrogen phosphate | CHEMBL1784786
Type:
Small organic molecule
Emp. Form.:
C49H81N16O20P
Mol. Mass.:
1245.2364
SMILES:
[#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8]P([#8])([#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r|