Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glutamate receptor ionotropic, kainate 3
Ligand
BDBM50088222
Substrate
n/a
Meas. Tech.
ChEBML_90598
Ki
617±n/a nM
Citation
 Baker, SR; Bleakman, D; Ezquerra, J; Ballyk, BA; Deverill, M; Ho, K; Kamboj, RK; Collado, I; Domínguez, C; Escribano, A; Mateo, AI; Pedregal, C; Rubio, A 4-Alkylidenyl glutamic acids, potent and selective GluR5 agonists. Bioorg Med Chem Lett 10:1807-10 (2000) [PubMed] Article
Baker, SR; Bleakman, D; Ezquerra, J; Ballyk, BA; Deverill, M; Ho, K; Kamboj, RK; Collado, I; Domínguez, C; Escribano, A; Mateo, AI; Pedregal, C; Rubio, A 4-Alkylidenyl glutamic acids, potent and selective GluR5 agonists. Bioorg Med Chem Lett 10:1807-10 (2000) [PubMed] Article More Info.:
Target
Name:
Glutamate receptor ionotropic, kainate 3
Synonyms:
EAA5 | Excitatory amino acid receptor 5 | GLUR7 | GRIK3 | GRIK3_HUMAN | GluR-7 | Glutamate receptor 7 | Glutamate receptor ionotropic kainate | Glutamate receptor ionotropic kainate 3 | Glutamate receptor, ionotropic kainate 3 | Glutamate-Kainate 7
Type:
Enzyme Catalytic Domain
Mol. Mass.:
104046.44
Organism:
Homo sapiens (Human)
Description:
Glutamate-Kainate 7 0 HUMAN::Q13003
Residue:
919
Sequence:
MTAPWRRLRSLVWEYWAGLLVCAFWIPDSRGMPHVIRIGGIFEYADGPNAQVMNAEEHAFRFSANIINRNRTLLPNTTLTYDIQRIHFHDSFEATKKACDQLALGVVAIFGPSQGSCTNAVQSICNALEVPHIQLRWKHHPLDNKDTFYVNLYPDYASLSHAILDLVQYLKWRSATVVYDDSTGLIRLQELIMAPSRYNIRLKIRQLPIDSDDSRPLLKEMKRGREFRIIFDCSHTMAAQILKQAMAMGMMTEYYHFIFTTLDLYALDLEPYRYSGVNLTGFRILNVDNPHVSAIVEKWSMERLQAAPRSESGLLDGVMMTDAALLYDAVHIVSVCYQRAPQMTVNSLQCHRHKAWRFGGRFMNFIKEAQWEGLTGRIVFNKTSGLRTDFDLDIISLKEDGLEKVGVWSPADGLNITEVAKGRGPNVTDSLTNRSLIVTTVLEEPFVMFRKSDRTLYGNDRFEGYCIDLLKELAHILGFSYEIRLVEDGKYGAQDDKGQWNGMVKELIDHKADLAVAPLTITHVREKAIDFSKPFMTLGVSILYRKPNGTNPSVFSFLNPLSPDIWMYVLLAYLGVSCVLFVIARFSPYEWYDAHPCNPGSEVVENNFTLLNSFWFGMGSLMQQGSELMPKALSTRIIGGIWWFFTLIIISSYTANLAAFLTVERMESPIDSADDLAKQTKIEYGAVKDGATMTFFKKSKISTFEKMWAFMSSKPSALVKNNEEGIQRALTADYALLMESTTIEYVTQRNCNLTQIGGLIDSKGYGIGTPMGSPYRDKITIAILQLQEEDKLHIMKEKWWRGSGCPEEENKEASALGIQKIGGIFIVLAAGLVLSVLVAVGEFVYKLRKTAEREQRSFCSTVADEIRFSLTCQRRVKHKPQPPMMVKTDAVINMHTFNDRRLPGKDSMACSTSLAPVFP
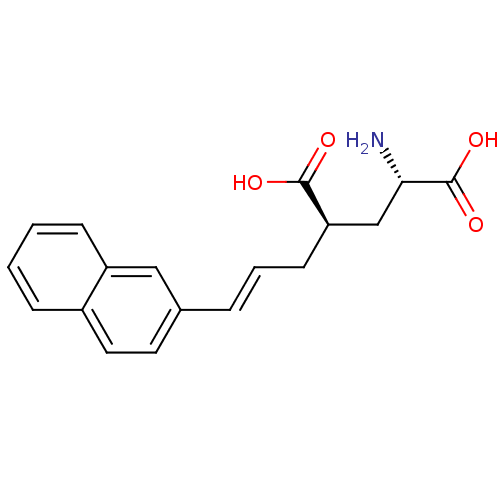
Inhibitor
Name:
BDBM50088222
Synonyms:
(2S,4R)-2-Amino-4-((E)-3-naphthalen-2-yl-allyl)-pentanedioic acid | CHEMBL301536 | E-2-Amino-4-(3-naphthalen-2-yl-allyl)-pentanedioic acid(LY339434) | LY-339434
Type:
Small organic molecule
Emp. Form.:
C18H19NO4
Mol. Mass.:
313.3478
SMILES:
N[C@@H](C[C@@H](C\C=C\c1ccc2ccccc2c1)C(O)=O)C(O)=O