Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Potassium-transporting ATPase alpha chain 2
Ligand
BDBM50131829
Substrate
n/a
Meas. Tech.
ChEMBL_144103 (CHEMBL751507)
IC50
6000±n/a nM
Citation
 De Munari, S; Cerri, A; Gobbini, M; Almirante, N; Banfi, L; Carzana, G; Ferrari, P; Marazzi, G; Micheletti, R; Schiavone, A; Sputore, S; Torri, M; Zappavigna, MP; Melloni, P Structure-based design and synthesis of novel potent Na+,K+ -ATPase inhibitors derived from a 5alpha,14alpha-androstane scaffold as positive inotropic compounds. J Med Chem 46:3644-54 (2003) [PubMed] Article
De Munari, S; Cerri, A; Gobbini, M; Almirante, N; Banfi, L; Carzana, G; Ferrari, P; Marazzi, G; Micheletti, R; Schiavone, A; Sputore, S; Torri, M; Zappavigna, MP; Melloni, P Structure-based design and synthesis of novel potent Na+,K+ -ATPase inhibitors derived from a 5alpha,14alpha-androstane scaffold as positive inotropic compounds. J Med Chem 46:3644-54 (2003) [PubMed] Article More Info.:
Target
Name:
Potassium-transporting ATPase alpha chain 2
Synonyms:
AT12A_HUMAN | ATP12A | ATP1AL1
Type:
PROTEIN
Mol. Mass.:
115509.17
Organism:
Homo sapiens (Human)
Description:
ChEMBL_144101
Residue:
1039
Sequence:
MHQKTPEIYSVELSGTKDIVKTDKGDGKEKYRGLKNNCLELKKKNHKEEFQKELHLDDHKLSNRELEEKYGTDIIMGLSSTRAAELLARDGPNSLTPPKQTPEIVKFLKQMVGGFSILLWVGAFLCWIAYGIQYSSDKSASLNNVYLGCVLGLVVILTGIFAYYQEAKSTNIMSSFNKMIPQQALVIRDSEKKTIPSEQLVVGDIVEVKGGDQIPADIRVLSSQGCRVDNSSLTGESEPQPRSSEFTHENPLETKNICFYSTTCLEGTVTGMVINTGDRTIIGHIASLASGVGNEKTPIAIEIEHFVHIVAGVAVSIGILFFIIAVSLKYQVLDSIIFLIGIIVANVPEGLLATVTVTLSLTAKRMAKKNCLVKNLEAVETLGSTSIICSDKTGTLTQNRMTVAHLWFDNQIFVADTSEDHSNQVFDQSSRTWASLSKIITLCNRAEFKPGQENVPIMKKAVIGDASETALLKFSEVILGDVMEIRKRNRKVAEIPFNSTNKFQLSIHEMDDPHGKRFLMVMKGAPERILEKCSTIMINGEEHPLDKSTAKTFHTAYMELGGLGERVLGFCHLYLPADEFPETYSFDIDAMNFPTSNLCFVGLLSMIDPPRSTVPDAVTKCRSAGIKVIMVTGDHPITAKAIAKSVGIISANSETVEDIAHRLNIAVEQVNKRDAKAAVVTGMELKDMSSEQLDEILANYQEIVFARTSPQQKLIIVEGCQRQDAVVAVTGDGVNDSPALKKADIGIAMGIAGSDAAKNAADMVLLDDNFASIVTGVEEGRLIFDNLKKTIAYSLTKNIAELCPFLIYIIVGLPLPIGTITILFIDLGTDIIPSIALAYEKAESDIMNRKPRHKNKDRLVNQPLAVYSYLHIGLMQALGAFLVYFTVYAQEGFLPRTLINLRVEWEKDYVNDLKDSYGQEWTRYQREYLEWTGYTAFFVGILVQQIADLIIRKTRRNSIFQQGLFRNKVIWVGITSQIIIGLILSYGLGSVTALSFTMLRAQYWFVAVPHAILIWVYDEVRKLFIRLYPGSWWDKNMYY
Inhibitor
Name:
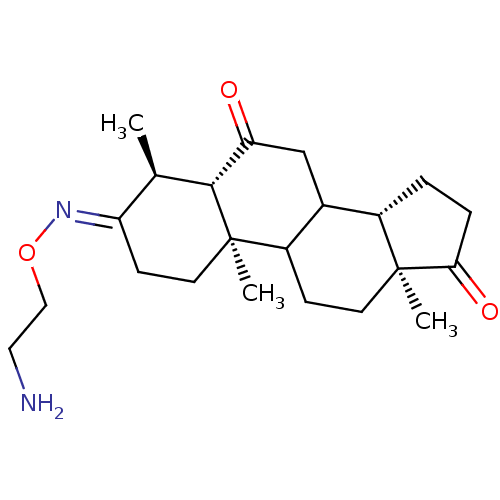
BDBM50131829
Synonyms:
(E)4,10,13-Trimethyl-tetradecahydro-cyclopenta[a]phenanthrene-3,6,17-trione 3-[O-(2-amino-ethyl)-oxime] | CHEMBL121572
Type:
Small organic molecule
Emp. Form.:
C22H34N2O3
Mol. Mass.:
374.517
SMILES:
C[C@H]1[C@@H]2C(=O)CC3[C@@H]4CCC(=O)[C@@]4(C)CCC3[C@@]2(C)CC\C1=N/OCCN