Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glycogen phosphorylase, muscle form
Ligand
BDBM50184615
Substrate
n/a
Meas. Tech.
ChEMBL_365330 (CHEMBL871240)
IC50
3300±n/a nM
Citation
More Info.:
Target
Name:
Glycogen phosphorylase, muscle form
Synonyms:
Glycogen Phosphorylase (PYGM) | Glycogen phosphorylase a (RMGPa) | Glycogen phosphorylase, muscle form | Myophosphorylase | PYGM | PYGM_RABIT
Type:
Enzyme
Mol. Mass.:
97296.32
Organism:
Oryctolagus cuniculus (rabbit)
Description:
Phosphorylation of Ser-15 converts phosphorylase B (unphosphorylated) to phosphorylase A.
Residue:
843
Sequence:
MSRPLSDQEKRKQISVRGLAGVENVTELKKNFNRHLHFTLVKDRNVATPRDYYFALAHTVRDHLVGRWIRTQQHYYEKDPKRIYYLSLEFYMGRTLQNTMVNLALENACDEATYQLGLDMEELEEIEEDAGLGNGGLGRLAACFLDSMATLGLAAYGYGIRYEFGIFNQKICGGWQMEEADDWLRYGNPWEKARPEFTLPVHFYGRVEHTSQGAKWVDTQVVLAMPYDTPVPGYRNNVVNTMRLWSAKAPNDFNLKDFNVGGYIQAVLDRNLAENISRVLYPNDNFFEGKELRLKQEYFVVAATLQDIIRRFKSSKFGCRDPVRTNFDAFPDKVAIQLNDTHPSLAIPELMRVLVDLERLDWDKAWEVTVKTCAYTNHTVLPEALERWPVHLLETLLPRHLQIIYEINQRFLNRVAAAFPGDVDRLRRMSLVEEGAVKRINMAHLCIAGSHAVNGVARIHSEILKKTIFKDFYELEPHKFQNKTNGITPRRWLVLCNPGLAEIIAERIGEEYISDLDQLRKLLSYVDDEAFIRDVAKVKQENKLKFAAYLEREYKVHINPNSLFDVQVKRIHEYKRQLLNCLHVITLYNRIKKEPNKFVVPRTVMIGGKAAPGYHMAKMIIKLITAIGDVVNHDPVVGDRLRVIFLENYRVSLAEKVIPAADLSEQISTAGTEASGTGNMKFMLNGALTIGTMDGANVEMAEEAGEENFFIFGMRVEDVDRLDQRGYNAQEYYDRIPELRQIIEQLSSGFFSPKQPDLFKDIVNMLMHHDRFKVFADYEEYVKCQERVSALYKNPREWTRMVIRNIATSGKFSSDRTIAQYAREIWGVEPSRQRLPAPDEKIP
Inhibitor
Name:
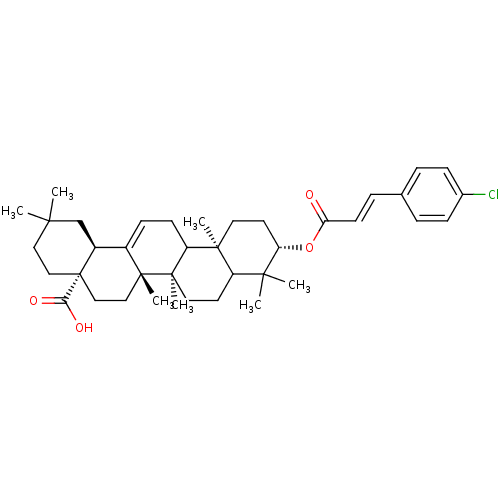
BDBM50184615
Synonyms:
(4aS,6aS,6bR,10S,12aR,14bS,E)-10-(3-(4-chlorophenyl)acryloyloxy)-2,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylic acid | CHEMBL150213
Type:
Small organic molecule
Emp. Form.:
C39H53ClO4
Mol. Mass.:
621.289
SMILES:
CC1(C)CC[C@@]2(CC[C@]3(C)C(=CCC4[C@@]5(C)CC[C@H](OC(=O)\C=C\c6ccc(Cl)cc6)C(C)(C)C5CC[C@@]34C)[C@@H]2C1)C(O)=O |c:10|