Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50329819
Substrate
n/a
Meas. Tech.
ChEMBL_464554 (CHEMBL933240)
IC50
3500±n/a nM
Citation
 Dorsey, BD; Iqbal, M; Chatterjee, S; Menta, E; Bernardini, R; Bernareggi, A; Cassarà, PG; D'Arasmo, G; Ferretti, E; De Munari, S; Oliva, A; Pezzoni, G; Allievi, C; Strepponi, I; Ruggeri, B; Ator, MA; Williams, M; Mallamo, JP Discovery of a potent, selective, and orally active proteasome inhibitor for the treatment of cancer. J Med Chem 51:1068-72 (2008) [PubMed] Article
Dorsey, BD; Iqbal, M; Chatterjee, S; Menta, E; Bernardini, R; Bernareggi, A; Cassarà, PG; D'Arasmo, G; Ferretti, E; De Munari, S; Oliva, A; Pezzoni, G; Allievi, C; Strepponi, I; Ruggeri, B; Ator, MA; Williams, M; Mallamo, JP Discovery of a potent, selective, and orally active proteasome inhibitor for the treatment of cancer. J Med Chem 51:1068-72 (2008) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
Inhibitor
Name:
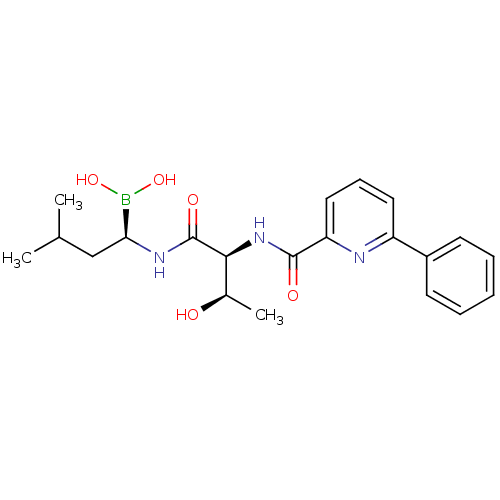
BDBM50329819
Synonyms:
(R)-1-((2S,3R)-3-hydroxy-2-(6-phenylpicolinamido)butanamido)-3-methylbutylboronic acid | CEP-18770 | CHEMBL270515 | [(1R)-1-[[(2S,3R)-3-hydroxy-2-[(6-phenylpyridine-2-carbonyl)amino]-1-oxobutyl]amino]-3-methylbutyl]boronic acid
Type:
Small organic molecule
Emp. Form.:
C21H28BN3O5
Mol. Mass.:
413.275
SMILES:
CC(C)C[C@H](NC(=O)[C@@H](NC(=O)c1cccc(n1)-c1ccccc1)[C@@H](C)O)B(O)O