Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glyoxalase I
Ligand
BDBM50092826
Substrate
n/a
Meas. Tech.
ChEMBL_571116 (CHEMBL1030416)
Ki
1150±n/a nM
Citation
More Info.:
Target
Name:
Glyoxalase I
Synonyms:
GLO1 | LGUL_YEAST | Lactoylglutathione lyase
Type:
PROTEIN
Mol. Mass.:
37210.98
Organism:
Saccharomyces cerevisiae
Description:
ChEMBL_571116
Residue:
326
Sequence:
MSTDSTRYPIQIEKASNDPTLLLNHTCLRVKDPARTVKFYTEHFGMKLLSRKDFEEAKFSLYFLSFPKDDIPKNKNGEPDVFSAHGVLELTHNWGTEKNPDYKINNGNEEPHRGFGHICFSVSDINKTCEELESQGVKFKKRLSEGRQKDIAFALGPDGYWIELITYSREGQEYPKGSVGNKFNHTMIRIKNPTRSLEFYQNVLGMKLLRTSEHESAKFTLYFLGYGVPKTDSVFSCESVLELTHNWGTENDPNFHYHNGNSEPQGYGHICISCDDAGALCKEIEVKYGDKIQWSPKFNQGRMKNIAFLKDPDGYSIEVVPHGLIA
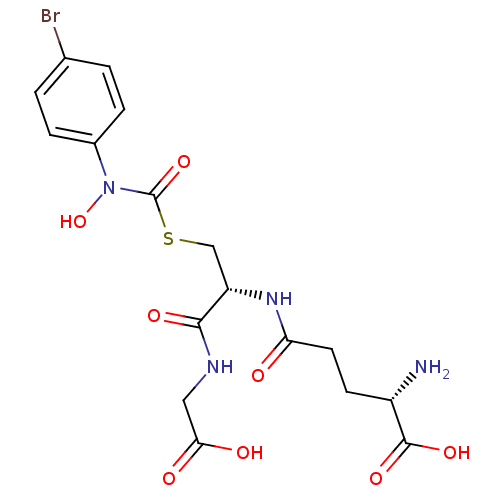
Inhibitor
Name:
BDBM50092826
Synonyms:
(2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydroxy)amino]carbonyl}thio)methyl]-2-(carboxyamino)-2-oxoethyl]amino}-5-oxopentanoic acid | (S)-2-amino-5-((R)-3-((4-bromophenyl)hydroxycarbamoylthio)-1-(carboxymethylamino)-1-oxopropan-2-ylamino)-5-oxopentanoic acid | CHEMBL128872 | S-(N-4bromophenyl-N-hydroxycarbamoyl)glutathione
Type:
Small organic molecule
Emp. Form.:
C17H21BrN4O8S
Mol. Mass.:
521.34
SMILES:
N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O