Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Fatty acid synthase
Ligand
BDBM50241343
Substrate
n/a
Meas. Tech.
ChEMBL_1467001 (CHEMBL3411198)
IC50
29600±n/a nM
Citation
More Info.:
Target
Name:
Fatty acid synthase
Synonyms:
FAS | FASN | FAS_HUMAN | Fatty acid synthase (FASN) | HU FASN
Type:
Enzyme
Mol. Mass.:
273419.37
Organism:
Homo sapiens (Human)
Description:
P49327
Residue:
2511
Sequence:
MEEVVIAGMSGKLPESENLQEFWDNLIGGVDMVTDDDRRWKAGLYGLPRRSGKLKDLSRFDASFFGVHPKQAHTMDPQLRLLLEVTYEAIVDGGINPDSLRGTHTGVWVGVSGSETSEALSRDPETLVGYSMVGCQRAMMANRLSFFFDFRGPSIALDTACSSSLMALQNAYQAIHSGQCPAAIVGGINVLLKPNTSVQFLRLGMLSPEGTCKAFDTAGNGYCRSEGVVAVLLTKKSLARRVYATILNAGTNTDGFKEQGVTFPSGDIQEQLIRSLYQSAGVAPESFEYIEAHGTGTKVGDPQELNGITRALCATRQEPLLIGSTKSNMGHPEPASGLAALAKVLLSLEHGLWAPNLHFHSPNPEIPALLDGRLQVVDQPLPVRGGNVGINSFGFGGSNVHIILRPNTQPPPAPAPHATLPRLLRASGRTPEAVQKLLEQGLRHSQDLAFLSMLNDIAAVPATAMPFRGYAVLGGERGGPEVQQVPAGERPLWFICSGMGTQWRGMGLSLMRLDRFRDSILRSDEAVKPFGLKVSQLLLSTDESTFDDIVHSFVSLTAIQIGLIDLLSCMGLRPDGIVGHSLGEVACGYADGCLSQEEAVLAAYWRGQCIKEAHLPPGAMAAVGLSWEECKQRCPPGVVPACHNSKDTVTISGPQAPVFEFVEQLRKEGVFAKEVRTGGMAFHSYFMEAIAPPLLQELKKVIREPKPRSARWLSTSIPEAQWHSSLARTSSAEYNVNNLVSPVLFQEALWHVPEHAVVLEIAPHALLQAVLKRGLKPSCTIIPLMKKDHRDNLEFFLAGIGRLHLSGIDANPNALFPPVEFPAPRGTPLISPLIKWDHSLAWDVPAAEDFPNGSGSPSAAIYNIDTSSESPDHYLVDHTLDGRVLFPATGYLSIVWKTLARALGLGVEQLPVVFEDVVLHQATILPKTGTVSLEVRLLEASRAFEVSENGNLVVSGKVYQWDDPDPRLFDHPESPTPNPTEPLFLAQAEVYKELRLRGYDYGPHFQGILEASLEGDSGRLLWKDNWVSFMDTMLQMSILGSAKHGLYLPTRVTAIHIDPATHRQKLYTLQDKAQVADVVVSRWLRVTVAGGVHISGLHTESAPRRQQEQQVPILEKFCFTPHTEEGCLSERAALQEELQLCKGLVQALQTKVTQQGLKMVVPGLDGAQIPRDPSQQELPRLLSAACRLQLNGNLQLELAQVLAQERPKLPEDPLLSGLLDSPALKACLDTAVENMPSLKMKVVEVLAGHGHLYSRIPGLLSPHPLLQLSYTATDRHPQALEAAQAELQQHDVAQGQWDPADPAPSALGSADLLVCNCAVAALGDPASALSNMVAALREGGFLLLHTLLRGHPLGDIVAFLTSTEPQYGQGILSQDAWESLFSRVSLRLVGLKKSFYGSTLFLCRRPTPQDSPIFLPVDDTSFRWVESLKGILADEDSSRPVWLKAINCATSGVVGLVNCLRREPGGNRLRCVLLSNLSSTSHVPEVDPGSAELQKVLQGDLVMNVYRDGAWGAFRHFLLEEDKPEEPTAHAFVSTLTRGDLSSIRWVCSSLRHAQPTCPGAQLCTVYYASLNFRDIMLATGKLSPDAIPGKWTSQDSLLGMEFSGRDASGKRVMGLVPAKGLATSVLLSPDFLWDVPSNWTLEEAASVPVVYSTAYYALVVRGRVRPGETLLIHSGSGGVGQAAIAIALSLGCRVFTTVGSAEKRAYLQARFPQLDSTSFANSRDTSFEQHVLWHTGGKGVDLVLNSLAEEKLQASVRCLATHGRFLEIGKFDLSQNHPLGMAIFLKNVTFHGVLLDAFFNESSADWREVWALVQAGIRDGVVRPLKCTVFHGAQVEDAFRYMAQGKHIGKVVVQVLAEEPEAVLKGAKPKLMSAISKTFCPAHKSYIIAGGLGGFGLELAQWLIQRGVQKLVLTSRSGIRTGYQAKQVRRWRRQGVQVQVSTSNISSLEGARGLIAEAAQLGPVGGVFNLAVVLRDGLLENQTPEFFQDVCKPKYSGTLNLDRVTREACPELDYFVVFSSVSCGRGNAGQSNYGFANSAMERICEKRRHEGLPGLAVQWGAIGDVGILVETMSTNDTIVSGTLPQRMASCLEVLDLFLNQPHMVLSSFVLAEKAAAYRDRDSQRDLVEAVAHILGIRDLAAVNLDSSLADLGLDSLMSVEVRQTLERELNLVLSVREVRQLTLRKLQELSSKADEASELACPTPKEDGLAQQQTQLNLRSLLVNPEGPTLMRLNSVQSSERPLFLVHPIEGSTTVFHSLASRLSIPTYGLQCTRAAPLDSIHSLAAYYIDCIRQVQPEGPYRVAGYSYGACVAFEMCSQLQAQQSPAPTHNSLFLFDGSPTYVLAYTQSYRAKLTPGCEAEAETEAICFFVQQFTDMEHNRVLEALLPLKGLEERVAAAVDLIIKSHQGLDRQELSFAARSFYYKLRAAEQYTPKAKYHGNVMLLRAKTGGAYGEDLGADYNLSQVCDGKVSVHVIEGDHRTLLEGSGLESIISIIHSSLAEPRVSVREG
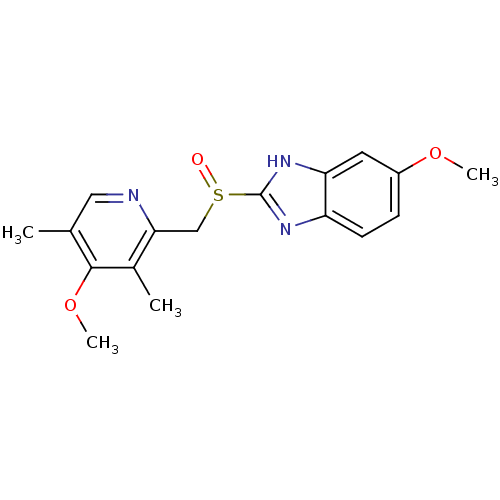
Inhibitor
Name:
BDBM50241343
Synonyms:
(RS)-6-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl) methylsulfinyl)-1H-benzo[d]imidazole | (omeprazole)5-Methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethanesulfinyl)-1H-benzoimidazole | 2-(3-methoxy-2,4-dimethylbenzylsulfinyl)-6-methoxy-1H-benzo[d]imidazole | 5-Methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethanesulfinyl)-1H-benzoimidazole | 5-Methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethanesulfinyl)-1H-benzoimidazole (omeprazole) | 5-Methoxy-2-(4-methoxy-3,5-dimethyl-pyridin-2-ylmethanesulfinyl)-1H-benzoimidazole(omeprazole) | 5-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl)-1H-benzo[d]imidazole | 5-methoxy-2-(2-(4-methoxy-3,5-dimethylpyridin-2-yl)ethylsulfinyl)-1H-benzo[d]imidazole | 6-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl)-1H-benzo[d]imidazole | CHEMBL1503 | ESOMEPRAZOLE SODIUM | H-168/68 | OMEPRAZOLE | Omeprazole (OMP) | Prilosec | cid_4594
Type:
Small organic molecule
Emp. Form.:
C17H19N3O3S
Mol. Mass.:
345.416
SMILES:
COc1ccc2nc([nH]c2c1)S(=O)Cc1ncc(C)c(OC)c1C