Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Histone deacetylase 6
Ligand
BDBM50101331
Substrate
n/a
Meas. Tech.
ChEMBL_1455100 (CHEMBL3366356)
IC50
61±n/a nM
Citation
 Salvador, LA; Park, H; Al-Awadhi, FH; Liu, Y; Kim, B; Zeller, SL; Chen, QY; Hong, J; Luesch, H Modulation of Activity Profiles for Largazole-Based HDAC Inhibitors through Alteration of Prodrug Properties. ACS Med Chem Lett 5:905-10 (2014) [PubMed] Article
Salvador, LA; Park, H; Al-Awadhi, FH; Liu, Y; Kim, B; Zeller, SL; Chen, QY; Hong, J; Luesch, H Modulation of Activity Profiles for Largazole-Based HDAC Inhibitors through Alteration of Prodrug Properties. ACS Med Chem Lett 5:905-10 (2014) [PubMed] Article More Info.:
Target
Name:
Histone deacetylase 6
Synonyms:
Cereblon/Histone deacetylase 6 | HD6 | HDAC6 | HDAC6_HUMAN | Histone deacetylase 6 (HDAC6) | Human HDAC6 | KIAA0901 | ORF Names:JM21
Type:
Chromatin regulator; hydrolase; repressor
Mol. Mass.:
131381.51
Organism:
Homo sapiens (Human)
Description:
Q9UBN7
Residue:
1215
Sequence:
MTSTGQDSTTTRQRRSRQNPQSPPQDSSVTSKRNIKKGAVPRSIPNLAEVKKKGKMKKLGQAMEEDLIVGLQGMDLNLEAEALAGTGLVLDEQLNEFHCLWDDSFPEGPERLHAIKEQLIQEGLLDRCVSFQARFAEKEELMLVHSLEYIDLMETTQYMNEGELRVLADTYDSVYLHPNSYSCACLASGSVLRLVDAVLGAEIRNGMAIIRPPGHHAQHSLMDGYCMFNHVAVAARYAQQKHRIRRVLIVDWDVHHGQGTQFTFDQDPSVLYFSIHRYEQGRFWPHLKASNWSTTGFGQGQGYTINVPWNQVGMRDADYIAAFLHVLLPVALEFQPQLVLVAAGFDALQGDPKGEMAATPAGFAQLTHLLMGLAGGKLILSLEGGYNLRALAEGVSASLHTLLGDPCPMLESPGAPCRSAQASVSCALEALEPFWEVLVRSTETVERDNMEEDNVEESEEEGPWEPPVLPILTWPVLQSRTGLVYDQNMMNHCNLWDSHHPEVPQRILRIMCRLEELGLAGRCLTLTPRPATEAELLTCHSAEYVGHLRATEKMKTRELHRESSNFDSIYICPSTFACAQLATGAACRLVEAVLSGEVLNGAAVVRPPGHHAEQDAACGFCFFNSVAVAARHAQTISGHALRILIVDWDVHHGNGTQHMFEDDPSVLYVSLHRYDHGTFFPMGDEGASSQIGRAAGTGFTVNVAWNGPRMGDADYLAAWHRLVLPIAYEFNPELVLVSAGFDAARGDPLGGCQVSPEGYAHLTHLLMGLASGRIILILEGGYNLTSISESMAACTRSLLGDPPPLLTLPRPPLSGALASITETIQVHRRYWRSLRVMKVEDREGPSSSKLVTKKAPQPAKPRLAERMTTREKKVLEAGMGKVTSASFGEESTPGQTNSETAVVALTQDQPSEAATGGATLAQTISEAAIGGAMLGQTTSEEAVGGATPDQTTSEETVGGAILDQTTSEDAVGGATLGQTTSEEAVGGATLAQTTSEAAMEGATLDQTTSEEAPGGTELIQTPLASSTDHQTPPTSPVQGTTPQISPSTLIGSLRTLELGSESQGASESQAPGEENLLGEAAGGQDMADSMLMQGSRGLTDQAIFYAVTPLPWCPHLVAVCPIPAAGLDVTQPCGDCGTIQENWVCLSCYQVYCGRYINGHMLQHHGNSGHPLVLSYIDLSAWCYYCQAYVHHQALLDVKNIAHQNKFGEDMPHPH
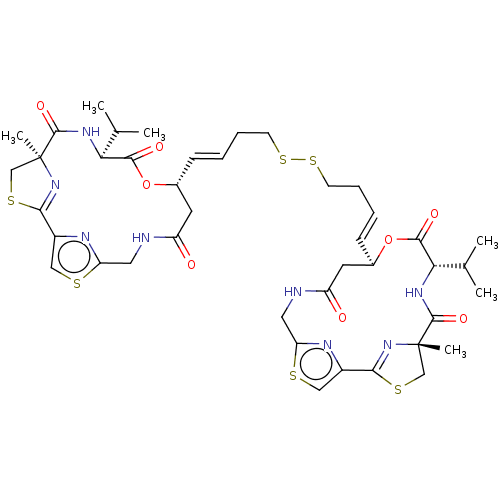
Inhibitor
Name:
BDBM50101331
Synonyms:
CHEMBL3329621
Type:
Small organic molecule
Emp. Form.:
C42H54N8O8S6
Mol. Mass.:
991.317
SMILES:
CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSCC\C=C\[C@@H]1CC(=O)NCc3nc(cs3)C3=N[C@@](C)(CS3)C(=O)N[C@@H](C(C)C)C(=O)O1)n2 |r,c:11,t:50|