Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Diacylglycerol lipase-alpha
Ligand
BDBM24567
Substrate
n/a
Meas. Tech.
ChEMBL_1553497 (CHEMBL3768668)
IC50
20±n/a nM
Citation
 Chupak, LS; Zheng, X; Hu, S; Huang, Y; Ding, M; Lewis, MA; Westphal, RS; Blat, Y; McClure, A; Gentles, RG Structure activity relationship studies on chemically non-reactive glycine sulfonamide inhibitors of diacylglycerol lipase. Bioorg Med Chem 24:1455-68 (2016) [PubMed] Article
Chupak, LS; Zheng, X; Hu, S; Huang, Y; Ding, M; Lewis, MA; Westphal, RS; Blat, Y; McClure, A; Gentles, RG Structure activity relationship studies on chemically non-reactive glycine sulfonamide inhibitors of diacylglycerol lipase. Bioorg Med Chem 24:1455-68 (2016) [PubMed] Article More Info.:
Target
Name:
Diacylglycerol lipase-alpha
Synonyms:
DGL-alpha | DGLA_MOUSE | Dagla | Kiaa0659 | Neural stem cell-derived dendrite regulator | Nsddr | Sn1-specific diacylglycerol lipase alpha
Type:
PROTEIN
Mol. Mass.:
115371.22
Organism:
Mus musculus
Description:
ChEMBL_101098
Residue:
1044
Sequence:
MPGIVVFRRRWSVGSDDLVLPAIFLFLLHTTWFVILSVVLFGLVYNPHEACSLNLVDHGRGYLGILLSCMIAEMAIIWLSMRGGILYTEPRDSMQYVLYVRLAILVIEFIYAIVGIVWLTQYYTSCNDLTAKNVTLGMVVCNWVVILSVCITVLCVFDPTGRTFVKLRATKRRQRNLRTYNLRHRLEEGQATSWSRRLKVFLCCTRTKDSQSDAYSEIAYLFAEFFRDLDIVPSDIIAGLVLLRQRQRAKRNAVLDEANNDILAFLSGMPVTRNTKYLDLKNSHEMLRYKEVCYYMLFALAAYGWPMYLMRKPTCGLCQLARSCSCCLCPARPRFAPGVTIEEDNCCGCNAIAIRRHFLDENMTAVDIVYTSCHDAVYETPFYVAVDHDKKKVVISIRGTLSPKDALTDLTGDAERLPVEGHRGTWLGHKGMVLSAEYIKKKLEQEMVLSQAFGRDLGRGTKHYGLIVVGHSLGAGTAAILSFLLRPQYPTLKCFAYSPPGGLLSEDAMEYSKEFVTAVVLGKDLVPRIGLSQLEGFRRQLLDVLQRSTKPKWRIIVGATKCIPKSELPEDQVEVTTLASTRLWTHPSDLTIALSASTPLYPPGRIIHVVHNHPAEQCCCCEQEEPTYFAIWGDNKAFNEVIISPAMLHEHLPYVVMEGLNKVLENYNKGKTALLSAAKVMVSPTEVDLTPELIFQQQPLPTGPPLPTGLALELPATEHRNSSVRSKSQSEMSLEGFSEGRLLSPVAAASAARQDPVELLLLSTQERLAAELQSRRAPLATMESLSDTESLYSFDSRRSSGFRSIRGSPSLHAVLERDEGHLFYIDPAIPEENPSLSSRTELLAADSLSKHSQDTQPLEAALGSGGVTPERPPSATIEEEEAAGGSEGGGVAPRGELALHNGRLGDSPSPQVLEFAEFIDSLFNLDSKSSSFQDLYCMMVPESPTSDYTEGPKSPSQQEILLRAQFEPNLVPKPPRLFAGSAEPSSGISLSPSFPLSSSGELMDLTPTGLSSQECLATDKIRTSTPTGHGASPTKQDDLVISAR
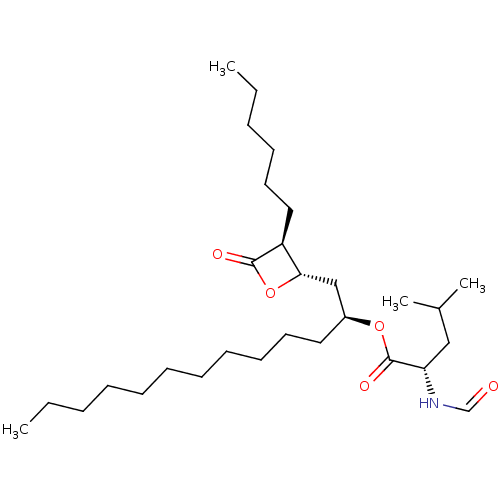
Inhibitor
Name:
BDBM24567
Synonyms:
(2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-2-yl (2S)-2-formamido-4-methylpentanoate | CHEMBL175247 | Orlistat | THLP | Tetrahydrolipstatin | US9328123, Orlistat | Xenical
Type:
Small organic molecule
Emp. Form.:
C29H53NO5
Mol. Mass.:
495.7348
SMILES:
CCCCCCCCCCC[C@@H](C[C@@H]1OC(=O)[C@H]1CCCCCC)OC(=O)[C@H](CC(C)C)NC=O |r|