Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Disintegrin and metalloproteinase domain-containing protein 17
Ligand
BDBM53380
Substrate
n/a
Meas. Tech.
QFRET-based biochemical high throughput dose response assay to identify exosite inhibitors of ADAM17
IC50
30390±n/a nM
Citation
 PubChem, PC QFRET-based biochemical high throughput dose response assay to identify exosite inhibitors of ADAM17 PubChem Bioassay (2014)[AID]
PubChem, PC QFRET-based biochemical high throughput dose response assay to identify exosite inhibitors of ADAM17 PubChem Bioassay (2014)[AID] More Info.:
Target
Name:
Disintegrin and metalloproteinase domain-containing protein 17
Synonyms:
ADA17_HUMAN | ADAM17 | CSVP | Disintegrin and metalloproteinase domain-containing protein 17 (ADAM-17) | Disintegrin and metalloproteinase domain-containing protein 17 (ADAM17) | TACE | TNF-alpha convertase | TNF-alpha converting enzyme (TACE) | TNF-alpha-converting enzyme (TACE) | Tumor Necrosis Factor Alpha Converting Enzyme | Tumor necrosis factor-α converting enzyme (TACE)
Type:
Enzyme
Mol. Mass.:
93007.89
Organism:
Homo sapiens (Human)
Description:
Residue:
824
Sequence:
MRQSLLFLTSVVPFVLAPRPPDDPGFGPHQRLEKLDSLLSDYDILSLSNIQQHSVRKRDLQTSTHVETLLTFSALKRHFKLYLTSSTERFSQNFKVVVVDGKNESEYTVKWQDFFTGHVVGEPDSRVLAHIRDDDVIIRINTDGAEYNIEPLWRFVNDTKDKRMLVYKSEDIKNVSRLQSPKVCGYLKVDNEELLPKGLVDREPPEELVHRVKRRADPDPMKNTCKLLVVADHRFYRYMGRGEESTTTNYLIELIDRVDDIYRNTSWDNAGFKGYGIQIEQIRILKSPQEVKPGEKHYNMAKSYPNEEKDAWDVKMLLEQFSFDIAEEASKVCLAHLFTYQDFDMGTLGLAYVGSPRANSHGGVCPKAYYSPVGKKNIYLNSGLTSTKNYGKTILTKEADLVTTHELGHNFGAEHDPDGLAECAPNEDQGGKYVMYPIAVSGDHENNKMFSNCSKQSIYKTIESKAQECFQERSNKVCGNSRVDEGEECDPGIMYLNNDTCCNSDCTLKEGVQCSDRNSPCCKNCQFETAQKKCQEAINATCKGVSYCTGNSSECPPPGNAEDDTVCLDLGKCKDGKCIPFCEREQQLESCACNETDNSCKVCCRDLSGRCVPYVDAEQKNLFLRKGKPCTVGFCDMNGKCEKRVQDVIERFWDFIDQLSINTFGKFLADNIVGSVLVFSLIFWIPFSILVHCVDKKLDKQYESLSLFHPSNVEMLSSMDSASVRIIKPFPAPQTPGRLQPAPVIPSAPAAPKLDHQRMDTIQEDPSTDSHMDEDGFEKDPFPNSSTAAKSFEDLTDHPVTRSEKAASFKLQRQNRVDSKETEC
Inhibitor
Name:
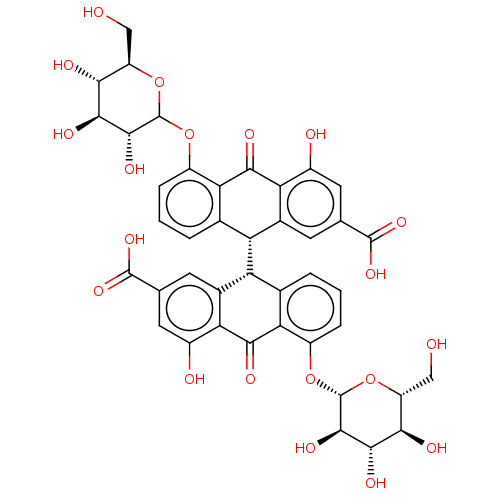
BDBM53380
Synonyms:
MLS001332477 | SMR000857110 | Sennoside A | cid_16218404
Type:
Small organic molecule
Emp. Form.:
C42H38O20
Mol. Mass.:
862.7391
SMILES:
[H][C@]1(c2cccc(OC3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c2C(=O)c2c(O)cc(cc12)C(O)=O)[C@]1([H])c2cccc(O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c2C(=O)c2c(O)cc(cc12)C(O)=O