Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
Ligand
BDBM124518
Substrate
n/a
Meas. Tech.
Enzyme Inhibition Assay
Temperature
298.15±n/a K
IC50
0.101±n/a nM
Comments
extracted
Citation
 Allen, JR; Biswas, K; Chavez, Jr., F; Chen, N; De Morin, FF; Falsey, JR; Frohn, MJ; Harrington, PE; Home, DB; Harrington, EH; Kaller, MR; Kunz, RK; Monenschein, H; Nguyen, TT; Pickrell, AJ; Reichelt, A; Rumfelt, S; Rzasa, RM; Sham, K; Yao, G Pyridine and pyrimidine derivatives as phosphodiesterase 10 inhibitors US Patent US8759532 Publication Date 6/24/2014
Allen, JR; Biswas, K; Chavez, Jr., F; Chen, N; De Morin, FF; Falsey, JR; Frohn, MJ; Harrington, PE; Home, DB; Harrington, EH; Kaller, MR; Kunz, RK; Monenschein, H; Nguyen, TT; Pickrell, AJ; Reichelt, A; Rumfelt, S; Rzasa, RM; Sham, K; Yao, G Pyridine and pyrimidine derivatives as phosphodiesterase 10 inhibitors US Patent US8759532 Publication Date 6/24/2014 More Info.:
Target
Name:
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
Synonyms:
3',5'-cyclic phosphodiesterase | 3.1.4.17 | PDE10A | PDE10_HUMAN | Phosphodiesterase 10 (PDE10) | Phosphodiesterase 10A
Type:
Protein
Mol. Mass.:
88412.52
Organism:
Homo sapiens (Human)
Description:
Q9Y233
Residue:
1055
Sequence:
MASLEEPLAPRPQGPLPAAGDEPGCGPGKLRPEPRLSAAGGGSAAGPGPAPEWPGRGRAERAAPPRPPLSSAGRPSPAGGPGALSARGGGCGWVAARAPLALAFSSRVPSSSPSFFYFWPPPPPPPPSFLPSSSAFHLPVRLPGREGAAAAAAAGGGGDAGGGGGGGQEAAPLSVPTSSSHRGGGGSGGGRRRLFLSPALQGLLLPARAGPRPPPPPRLPLGQAARRAGSPGFPGAGPGGGGQTPRRPQGASFALAAAAALLFGSDMEDGPSNNASCFRRLTECFLSPSLTDEKVKAYLSLHPQVLDEFVSESVSAETVEKWLKRKNNKSEDESAPKEVSRYQDTNMQGVVYELNSYIEQRLDTGGDNQLLLYELSSIIKIATKADGFALYFLGECNNSLCIFTPPGIKEGKPRLIPAGPITQGTTVSAYVAKSRKTLLVEDILGDERFPRGTGLESGTRIQSVLCLPIVTAIGDLIGILELYRHWGKEAFCLSHQEVATANLAWASVAIHQVQVCRGLAKQTELNDFLLDVSKTYFDNIVAIDSLLEHIMIYAKNLVNADRCALFQVDHKNKELYSDLFDIGEEKEGKPVFKKTKEIRFSIEKGIAGQVARTGEVLNIPDAYADPRFNREVDLYTGYTTRNILCMPIVSRGSVIGVVQMVNKISGSAFSKTDENNFKMFAVFCALALHCANMYHRIRHSECIYRVTMEKLSYHSICTSEEWQGLMQFTLPVRLCKEIELFHFDIGPFENMWPGIFVYMVHRSCGTSCFELEKLCRFIMSVKKNYRRVPYHNWKHAVTVAHCMYAILQNNHTLFTDLERKGLLIACLCHDLDHRGFSNSYLQKFDHPLAALYSTSTMEQHHFSQTVSILQLEGHNIFSTLSSSEYEQVLEIIRKAIIATDLALYFGNRKQLEEMYQTGSLNLNNQSHRDRVIGLMMTACDLCSVTKLWPVTKLTANDIYAEFWAEGDEMKKLGIQPIPMMDRDKKDEVPQGQLGFYNAVAIPCYTTLTQILPPTEPLLKACRDNLSQWEKVIRGEETATWISSPSVAQKAAASED
Inhibitor
Name:
BDBM124518
Synonyms:
US8759532, 195
Type:
Small organic molecule
Emp. Form.:
C25H23N3O3
Mol. Mass.:
413.4684
SMILES:
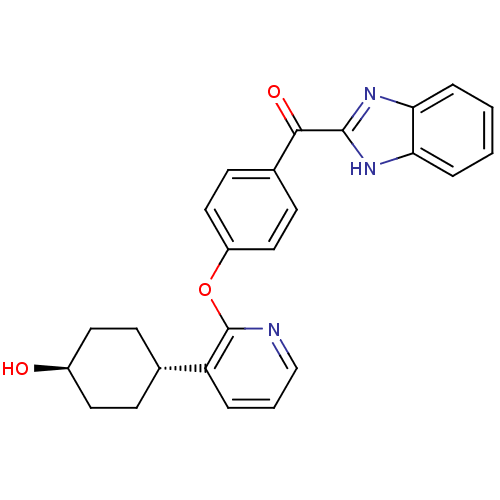
O[C@H]1CC[C@@H](CC1)c1cccnc1Oc1ccc(cc1)C(=O)c1nc2ccccc2[nH]1 |r,wU:4.7,wD:1.0,(-6.37,5.39,;-6.37,3.85,;-7.71,3.08,;-7.71,1.54,;-6.37,.77,;-5.04,1.54,;-5.04,3.08,;-6.37,-.77,;-7.71,-1.54,;-7.71,-3.08,;-6.37,-3.85,;-5.04,-3.08,;-5.04,-1.54,;-3.71,-.77,;-2.37,-1.54,;-2.37,-3.08,;-1.04,-3.85,;.29,-3.08,;.29,-1.54,;-1.04,-.77,;1.63,-3.85,;1.63,-5.39,;2.96,-3.08,;3.12,-1.55,;4.63,-1.23,;5.4,.11,;6.94,.11,;7.71,-1.23,;6.94,-2.56,;5.4,-2.56,;4.37,-3.71,)|