Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Carboxylic ester hydrolase
Ligand
BDBM175405
Substrate
n/a
Meas. Tech.
AChE and BChE Inhibition Activity
pH
8±n/a
IC50
3.65e+4± 1.19e+3 nM
Comments
extracted
Citation
 Hameed, A; Zehra, ST; Abbas, S; Nisa, RU; Mahmood, T; Ayub, K; Al-Rashida, M; Bajorath, J; Khan, KM; Iqbal, J One-pot synthesis of tetrazole-1,2,5,6-tetrahydronicotinonitriles and cholinesterase inhibition: Probing the plausible reaction mechanism via computational studies. Bioorg Chem 65:38-47 (2016) [PubMed] Article
Hameed, A; Zehra, ST; Abbas, S; Nisa, RU; Mahmood, T; Ayub, K; Al-Rashida, M; Bajorath, J; Khan, KM; Iqbal, J One-pot synthesis of tetrazole-1,2,5,6-tetrahydronicotinonitriles and cholinesterase inhibition: Probing the plausible reaction mechanism via computational studies. Bioorg Chem 65:38-47 (2016) [PubMed] Article More Info.:
Target
Name:
Carboxylic ester hydrolase
Synonyms:
BuChE | Butyrlcholinesterase (BuChE) | Butyrylcholine esterase | Butyrylcholinesterase | Butyrylcholinesterase (BChE) | Butyrylcholinesterase (BuChE) | Butyrylcholinesterase (EqBuChE) | Carboxylic ester hydrolase | butyrylcholinesterase precursor
Type:
Protein
Mol. Mass.:
68842.83
Organism:
Equus caballus (Horse)
Description:
Q9N1N9
Residue:
602
Sequence:
MQSWGTIICIRILLRFLLLWVLIGNSHTEEDIIITTKNGKVRGMNLPVLGGTVTAFLGIPYAQPPLGRLRFKKPQSLTKWSNIWNATKYANSCYQNTDQSFPGFLGSEMWNPNTELSEDCLYLNVWIPAPKPKNATVMIWIYGGGFQTGTSSLPVYDGKFLARVERVIVVSMNYRVGALGFLALSENPEAPGNMGLFDQQLALQWVQKNIAAFGGNPRSVTLFGESAGAASVSLHLLSPRSQPLFTRAILQSGSSNAPWAVTSLYEARNRTLTLAKRMGCSRDNETEMIKCLRDKDPQEILLNEVFVVPYDTLLSVNFGPTVDGDFLTDMPDTLLQLGQFKRTQILVGVNKDEGTAFLVYGAPGFSKDNNSIITRKEFQEGLKIFFPRVSEFGRESILFHYMDWLDDQRAENYREALDDVVGDYNIICPALEFTKKFSELGNDAFFYYFEHRSTKLPWPEWMGVMHGYEIEFVFGLPLERRVNYTKAEEILSRSIMKRWANFAKYGNPNGTQSNSTRWPVFKSTEQKYLTLNTESPKVYTKLRAQQCRFWTLFFPKVLELTGNIDEAEREWKAGFHRWNNYMMDWKNQFNDYTSKKESCSDF
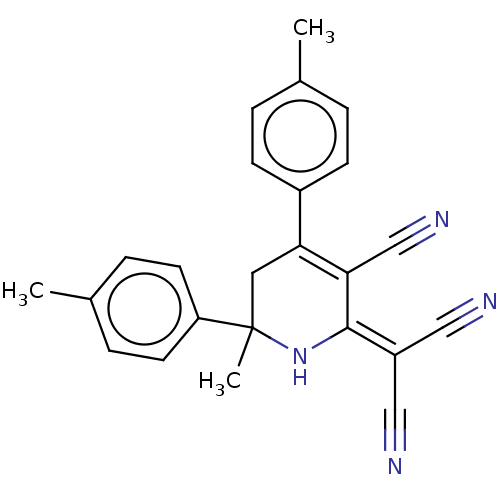
Inhibitor
Name:
BDBM175405
Synonyms:
2-[3-cyano-6-methyl-4,6-bis(4-methylphenyl)-1,2,5,6-tetrahydropyridin-2-ylidene]propanedinitrile (5b)
Type:
Small organic molecule
Emp. Form.:
C24H20N4
Mol. Mass.:
364.4424
SMILES:
[#6]-c1ccc(cc1)-[#6]-1=[#6](C#N)\[#6](-[#7]C([#6])([#6]-1)c1ccc(-[#6])cc1)=[#6](/C#N)C#N |c:8|