Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Lysine-specific demethylase 5A
Ligand
BDBM60875
Substrate
n/a
Meas. Tech.
KDM5 TR-FRET Assay
pH
7±n/a
IC50
2900±n/a nM
Comments
extracted
Citation
 Vinogradova, M; Gehling, VS; Gustafson, A; Arora, S; Tindell, CA; Wilson, C; Williamson, KE; Guler, GD; Gangurde, P; Manieri, W; Busby, J; Flynn, EM; Lan, F; Kim, HJ; Odate, S; Cochran, AG; Liu, Y; Wongchenko, M; Yang, Y; Cheung, TK; Maile, TM; Lau, T; Costa, M; Hegde, GV; Jackson, E; Pitti, R; Arnott, D; Bailey, C; Bellon, S; Cummings, RT; Albrecht, BK; Harmange, JC; Kiefer, JR; Trojer, P; Classon, M An inhibitor of KDM5 demethylases reduces survival of drug-tolerant cancer cells. Nat Chem Biol 12:531-8 (2016) [PubMed] Article
Vinogradova, M; Gehling, VS; Gustafson, A; Arora, S; Tindell, CA; Wilson, C; Williamson, KE; Guler, GD; Gangurde, P; Manieri, W; Busby, J; Flynn, EM; Lan, F; Kim, HJ; Odate, S; Cochran, AG; Liu, Y; Wongchenko, M; Yang, Y; Cheung, TK; Maile, TM; Lau, T; Costa, M; Hegde, GV; Jackson, E; Pitti, R; Arnott, D; Bailey, C; Bellon, S; Cummings, RT; Albrecht, BK; Harmange, JC; Kiefer, JR; Trojer, P; Classon, M An inhibitor of KDM5 demethylases reduces survival of drug-tolerant cancer cells. Nat Chem Biol 12:531-8 (2016) [PubMed] Article More Info.:
Target
Name:
Lysine-specific demethylase 5A
Synonyms:
Histone demethylase JARID1A | JARID1A | Jumonji/ARID domain-containing protein 1A | KDM5A | KDM5A_HUMAN | Lysine-specific demethylase 5A (KDM5A) | RBBP-2 | RBBP2 | RBP2 | Retinoblastoma-binding protein 2
Type:
Protein
Mol. Mass.:
192091.79
Organism:
Homo sapiens (Human)
Description:
P29375
Residue:
1690
Sequence:
MAGVGPGGYAAEFVPPPECPVFEPSWEEFTDPLSFIGRIRPLAEKTGICKIRPPKDWQPPFACEVKSFRFTPRVQRLNELEAMTRVRLDFLDQLAKFWELQGSTLKIPVVERKILDLYALSKIVASKGGFEMVTKEKKWSKVGSRLGYLPGKGTGSLLKSHYERILYPYELFQSGVSLMGVQMPNLDLKEKVEPEVLSTDTQTSPEPGTRMNILPKRTRRVKTQSESGDVSRNTELKKLQIFGAGPKVVGLAMGTKDKEDEVTRRRKVTNRSDAFNMQMRQRKGTLSVNFVDLYVCMFCGRGNNEDKLLLCDGCDDSYHTFCLIPPLPDVPKGDWRCPKCVAEECSKPREAFGFEQAVREYTLQSFGEMADNFKSDYFNMPVHMVPTELVEKEFWRLVSSIEEDVIVEYGADISSKDFGSGFPVKDGRRKILPEEEEYALSGWNLNNMPVLEQSVLAHINVDISGMKVPWLYVGMCFSSFCWHIEDHWSYSINYLHWGEPKTWYGVPSHAAEQLEEVMRELAPELFESQPDLLHQLVTIMNPNVLMEHGVPVYRTNQCAGEFVVTFPRAYHSGFNQGYNFAEAVNFCTADWLPIGRQCVNHYRRLRRHCVFSHEELIFKMAADPECLDVGLAAMVCKELTLMTEEETRLRESVVQMGVLMSEEEVFELVPDDERQCSACRTTCFLSALTCSCNPERLVCLYHPTDLCPCPMQKKCLRYRYPLEDLPSLLYGVKVRAQSYDTWVSRVTEALSANFNHKKDLIELRVMLEDAEDRKYPENDLFRKLRDAVKEAETCASVAQLLLSKKQKHRQSPDSGRTRTKLTVEELKAFVQQLFSLPCVISQARQVKNLLDDVEEFHERAQEAMMDETPDSSKLQMLIDMGSSLYVELPELPRLKQELQQARWLDEVRLTLSDPQQVTLDVMKKLIDSGVGLAPHHAVEKAMAELQELLTVSERWEEKAKVCLQARPRHSVASLESIVNEAKNIPAFLPNVLSLKEALQKAREWTAKVEAIQSGSNYAYLEQLESLSAKGRPIPVRLEALPQVESQVAAARAWRERTGRTFLKKNSSHTLLQVLSPRTDIGVYGSGKNRRKKVKELIEKEKEKDLDLEPLSDLEEGLEETRDTAMVVAVFKEREQKEIEAMHSLRAANLAKMTMVDRIEEVKFCICRKTASGFMLQCELCKDWFHNSCVPLPKSSSQKKGSSWQAKEVKFLCPLCMRSRRPRLETILSLLVSLQKLPVRLPEGEALQCLTERAMSWQDRARQALATDELSSALAKLSVLSQRMVEQAAREKTEKIISAELQKAAANPDLQGHLPSFQQSAFNRVVSSVSSSPRQTMDYDDEETDSDEDIRETYGYDMKDTASVKSSSSLEPNLFCDEEIPIKSEEVVTHMWTAPSFCAEHAYSSASKSCSQGSSTPRKQPRKSPLVPRSLEPPVLELSPGAKAQLEELMMVGDLLEVSLDETQHIWRILQATHPPSEDRFLHIMEDDSMEEKPLKVKGKDSSEKKRKRKLEKVEQLFGEGKQKSKELKKMDKPRKKKLKLGADKSKELNKLAKKLAKEEERKKKKEKAAAAKVELVKESTEKKREKKVLDIPSKYDWSGAEESDDENAVCAAQNCQRPCKDKVDWVQCDGGCDEWFHQVCVGVSPEMAENEDYICINCAKKQGPVSPGPAPPPSFIMSYKLPMEDLKETS
Inhibitor
Name:
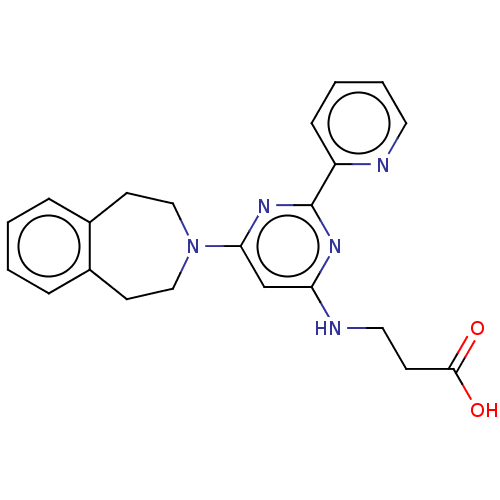
BDBM60875
Synonyms:
3-((6-(4,5-Dihydro-1H-benzo[d]azepin-3(2H)-yl)-2-(pyridin-2-yl)pyrimidin-4-yl)amino)propanoic acid | 3-{[2-(pyridin-2-yl)-6-(2,3,4,5-tetrahydro-1H-3-benzazepin-3-yl)pyrimidin-4-yl]amino}propanoic acid | GSK J1 | GSK-J1 | GSKJ1
Type:
n/a
Emp. Form.:
C22H23N5O2
Mol. Mass.:
389.4503
SMILES:
OC(=O)CCNc1cc(nc(n1)-c1ccccn1)N1CCc2ccccc2CC1