Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
UDP-glucuronosyltransferase 2B10
Ligand
BDBM234401
Substrate
Diclofenac
Meas. Tech.
UDP-glucuronosyltransferase Activity Assay
IC50
>3.00e+5±n/a nM
Citation
More Info.:
Target
Name:
UDP-glucuronosyltransferase 2B10
Synonyms:
UDB10_HUMAN | UGT2B10 | Uridine-5'-diphosphoglucuronosyltransferase 2B10
Type:
Enzyme
Mol. Mass.:
60792.40
Organism:
Homo sapiens (Human)
Description:
P36537
Residue:
528
Sequence:
MALKWTTVLLIQLSFYFSSGSCGKVLVWAAEYSLWMNMKTILKELVQRGHEVTVLASSASILFDPNDSSTLKLEVYPTSLTKTEFENIIMQLVKRLSEIQKDTFWLPFSQEQEILWAINDIIRNFCKDVVSNKKLMKKLQESRFDIVFADAYLPCGELLAELFNIPFVYSHSFSPGYSFERHSGGFIFPPSYVPVVMSKLSDQMTFMERVKNMLYVLYFDFWFQIFNMKKWDQFYSEVLGRPTTLSETMRKADIWLMRNSWNFKFPHPFLPNVDFVGGLHCKPAKPLPKEMEEFVQSSGENGVVVFSLGSMVSNMTEERANVIATALAKIPQKVLWRFDGNKPDALGLNTRLYKWIPQNDLLGHPKTRAFITHGGANGIYEAIYHGIPMVGIPLFFDQPDNIAHMKAKGAAVRVDFNTMSSTDLLNALKTVINDPSYKENIMKLSRIQHDQPVKPLDRAVFWIEFVMRHKGAKHLRVAAHNLTWFQYHSLDVIGFLLACVATVLFIITKCCLFCFWKFARKGKKGKRD
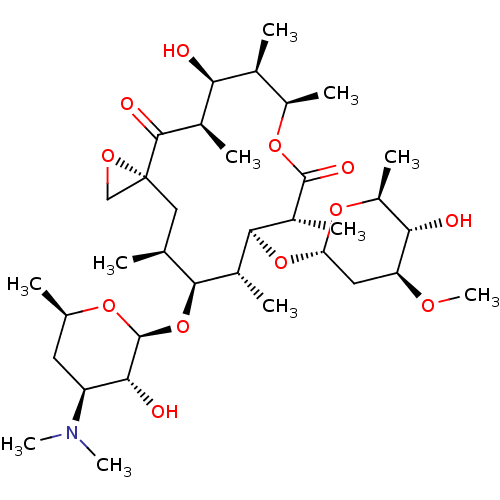
Inhibitor
Name:
BDBM234401
Synonyms:
Oleandomycin
Type:
Small organic molecule
Emp. Form.:
C35H61NO12
Mol. Mass.:
687.8583
SMILES:
CO[C@H]1C[C@H](O[C@H]2[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@@H](C)C[C@@]3(CO3)C(=O)[C@H](C)[C@@H](O)[C@@H](C)[C@@H](C)OC(=O)[C@@H]2C)O[C@@H](C)[C@@H]1O
Substrate
Name:
BDBM13066
Synonyms:
2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid | CHEMBL139 | Diclofenac | US11337935, Compound Diclofenac | US11478464, Compound Diclofenac | US11786535, Compound Diclofenac | {2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid
Type:
Small organic molecule
Emp. Form.:
C14H11Cl2NO2
Mol. Mass.:
296.149
SMILES:
OC(=O)Cc1ccccc1Nc1c(Cl)cccc1Cl