Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Neuraminidase
Ligand
BDBM4994
Substrate
n/a
Meas. Tech.
NA Inhibition Assay
IC50
170±n/a nM
Citation
More Info.:
Target
Name:
Neuraminidase
Synonyms:
NA | NRAM_I82A7 | Neuraminidase (NA)
Type:
Enzyme
Mol. Mass.:
49917.65
Organism:
Influenza A virus H3N2
Description:
Q67344
Residue:
446
Sequence:
MQIAILVTTVTLHFNQYECDSLADNQVMPCEPIIIERNITEIIYLTNTTIEKEICPKLMEYRNWSRPQCKITGFAPFSKDNSIRLSAGGDIWVTREPYVSCDPGKCYQFALGQGTTLDNKHSNDTIHDRIPHRTLLMNELGVPFHLGTRQVCIAWSSSSCHDGKAWLHVCVTGDDKNATASFIYDGRLVDSMGSWSQNILRTQESECVCINGTCTVVMTDGSASGRADTRILFIEEGKIVHISPLSGSAQHVEECSCYPRYPSVRCICRDNWKGSNRPIVDINIKDYSIDSRYVCSGLVGDTPRNNDRSSSSDCKNPNNDKGNHGVKGWAFDDGNDVWMGRTISKDSRSGYETFKVIDGWSTPNSKSQINRQVIVDRDNRSGYSGIFSVESKGCINRCFYVELIRGRKQETRVWWTSSSIVVFCGTSGTYGKGSWPDGANINFMPI
Inhibitor
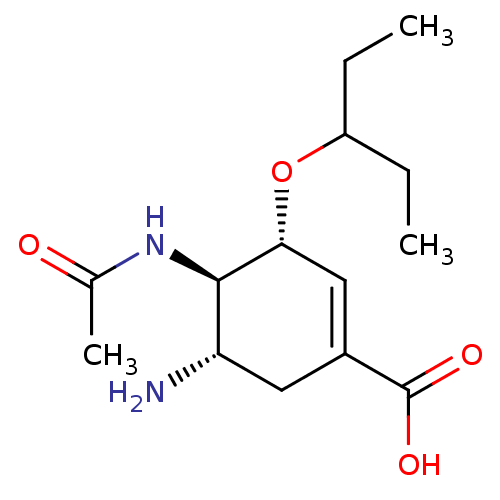
Name:
BDBM4994
Synonyms:
(3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylic acid | CHEMBL674 | GS4071 | Oseltamivir carboxylate
Type:
Small organic molecule
Emp. Form.:
C14H24N2O4
Mol. Mass.:
284.3514
SMILES:
CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7|