Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Endothelial lipase
Ligand
BDBM254876
Substrate
n/a
Meas. Tech.
Enzymatic Assay
pH
8±n/a
IC50
9919.00±n/a nM
Comments
extracted
Citation
More Info.:
Target
Name:
Endothelial lipase
Synonyms:
EDL | EL | Endothelial cell-derived lipase | LIPE_HUMAN | LIPG
Type:
Protein
Mol. Mass.:
56805.62
Organism:
Homo sapiens (Human)
Description:
Q9Y5X9
Residue:
500
Sequence:
MSNSVPLLCFWSLCYCFAAGSPVPFGPEGRLEDKLHKPKATQTEVKPSVRFNLRTSKDPEHEGCYLSVGHSQPLEDCSFNMTAKTFFIIHGWTMSGIFENWLHKLVSALHTREKDANVVVVDWLPLAHQLYTDAVNNTRVVGHSIARMLDWLQEKDDFSLGNVHLIGYSLGAHVAGYAGNFVKGTVGRITGLDPAGPMFEGADIHKRLSPDDADFVDVLHTYTRSFGLSIGIQMPVGHIDIYPNGGDFQPGCGLNDVLGSIAYGTITEVVKCEHERAVHLFVDSLVNQDKPSFAFQCTDSNRFKKGICLSCRKNRCNSIGYNAKKMRNKRNSKMYLKTRAGMPFRVYHYQMKIHVFSYKNMGEIEPTFYVTLYGTNADSQTLPLEIVERIEQNATNTFLVYTEEDLGDLLKIQLTWEGASQSWYNLWKEFRSYLSQPRNPGRELNIRRIRVKSGETQRKLTFCTEDPENTSISPGRELWFRKCRDGWRMKNETSPTVELP
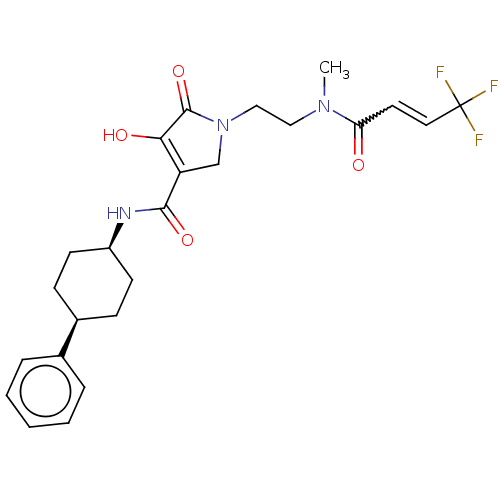
Inhibitor
Name:
BDBM254876
Synonyms:
US9493412, 178
Type:
Small organic molecule
Emp. Form.:
C24H28F3N3O4
Mol. Mass.:
479.492
SMILES:
CN(CCN1CC(C(=O)N[C@H]2CC[C@H](CC2)c2ccccc2)=C(O)C1=O)C(=O)C=CC(F)(F)F |w:28.30,wU:13.16,10.9,t:23,(8.06,-3.53,;8.06,-1.99,;6.73,-1.22,;5.39,-1.99,;4.06,-1.22,;2.65,-1.84,;1.62,-.7,;.09,-.86,;-.82,.39,;-.54,-2.27,;-2.07,-2.43,;-2.69,-3.84,;-4.23,-4,;-5.13,-2.75,;-4.5,-1.34,;-2.97,-1.18,;-6.66,-2.91,;-7.29,-4.32,;-8.82,-4.48,;-9.73,-3.23,;-9.1,-1.83,;-7.57,-1.67,;2.39,.63,;1.76,2.04,;3.9,.31,;5.04,1.34,;9.39,-1.22,;9.39,.32,;10.73,-1.99,;10.73,-3.53,;12.06,-4.3,;13.39,-5.07,;12.83,-2.96,;11.29,-5.63,)|