Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Proto-oncogene tyrosine-protein kinase receptor Ret [V804M]
Ligand
BDBM473712
Substrate
n/a
Meas. Tech.
Time-Resolved Fluorescence Transfer (FRET) Assay
IC50
46.2±n/a nM
Citation
 Jordan, AM; Newton, R; Waszkowycz, B; Sutton, JM; Hynd, G; Paoletta, S; Fordyce, EA Heterocyclic compounds as RET kinase inhibitors US Patent US10844067 Publication Date 11/24/2020
Jordan, AM; Newton, R; Waszkowycz, B; Sutton, JM; Hynd, G; Paoletta, S; Fordyce, EA Heterocyclic compounds as RET kinase inhibitors US Patent US10844067 Publication Date 11/24/2020 More Info.:
Target
Name:
Proto-oncogene tyrosine-protein kinase receptor Ret [V804M]
Synonyms:
CDHF12 | CDHR16 | PTC | Proto-oncogene tyrosine-protein kinase receptor Ret (V804M) | RET | RET kinase mutant (V804M) | RET51 | RET_HUMAN | ret enzyme (v804m)
Type:
Enzyme Catalytic Domain
Mol. Mass.:
124350.36
Organism:
Homo sapiens (Human)
Description:
P07949[V804M]
Residue:
1114
Sequence:
MAKATSGAAGLRLLLLLLLPLLGKVALGLYFSRDAYWEKLYVDQAAGTPLLYVHALRDAPEEVPSFRLGQHLYGTYRTRLHENNWICIQEDTGLLYLNRSLDHSSWEKLSVRNRGFPLLTVYLKVFLSPTSLREGECQWPGCARVYFSFFNTSFPACSSLKPRELCFPETRPSFRIRENRPPGTFHQFRLLPVQFLCPNISVAYRLLEGEGLPFRCAPDSLEVSTRWALDREQREKYELVAVCTVHAGAREEVVMVPFPVTVYDEDDSAPTFPAGVDTASAVVEFKRKEDTVVATLRVFDADVVPASGELVRRYTSTLLPGDTWAQQTFRVEHWPNETSVQANGSFVRATVHDYRLVLNRNLSISENRTMQLAVLVNDSDFQGPGAGVLLLHFNVSVLPVSLHLPSTYSLSVSRRARRFAQIGKVCVENCQAFSGINVQYKLHSSGANCSTLGVVTSAEDTSGILFVNDTKALRRPKCAELHYMVVATDQQTSRQAQAQLLVTVEGSYVAEEAGCPLSCAVSKRRLECEECGGLGSPTGRCEWRQGDGKGITRNFSTCSPSTKTCPDGHCDVVETQDINICPQDCLRGSIVGGHEPGEPRGIKAGYGTCNCFPEEEKCFCEPEDIQDPLCDELCRTVIAAAVLFSFIVSVLLSAFCIHCYHKFAHKPPISSAEMTFRRPAQAFPVSYSSSGARRPSLDSMENQVSVDAFKILEDPKWEFPRKNLVLGKTLGEGEFGKVVKATAFHLKGRAGYTTVAVKMLKENASPSELRDLLSEFNVLKQVNHPHVIKLYGACSQDGPLLLIMEYAKYGSLRGFLRESRKVGPGYLGSGGSRNSSSLDHPDERALTMGDLISFAWQISQGMQYLAEMKLVHRDLAARNILVAEGRKMKISDFGLSRDVYEEDSYVKRSQGRIPVKWMAIESLFDHIYTTQSDVWSFGVLLWEIVTLGGNPYPGIPPERLFNLLKTGHRMERPDNCSEEMYRLMLQCWKQEPDKRPVFADISKDLEKMMVKRRDYLDLAASTPSDSLIYDDGLSEEETPLVDCNNAPLPRALPSTWIENKLYGMSDPNWPGESPVPLTRADGTNTGFPRYPNDSVYANWMLSPSAAKLMDTFDS
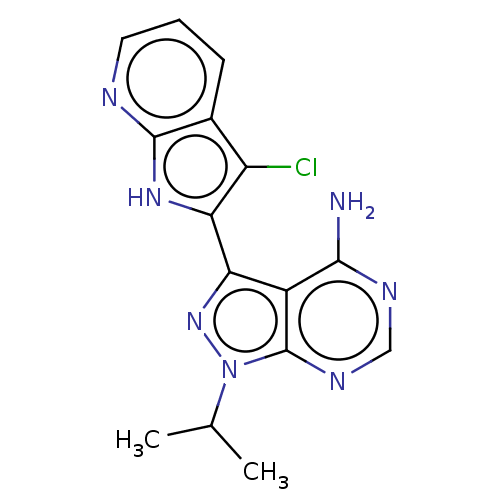
Inhibitor
Name:
BDBM473712
Synonyms:
3-(3-Chloro-1H-pyrrolo[2,3-b]pyridin-2-yl)- 1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-4- amine | US10844067, Example 55 | US11661423, Example 55
Type:
Small organic molecule
Emp. Form.:
C15H14ClN7
Mol. Mass.:
327.772
SMILES:
CC(C)n1nc(-c2[nH]c3ncccc3c2Cl)c2c(N)ncnc12