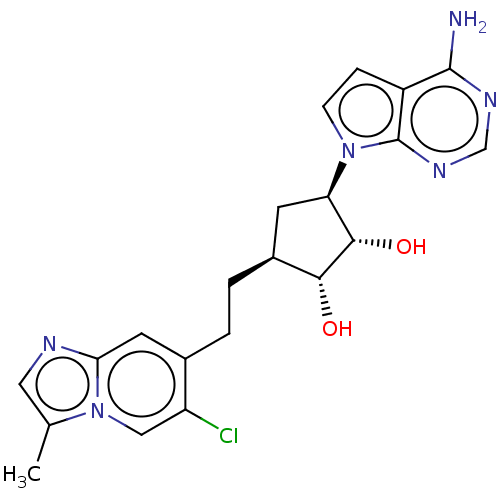
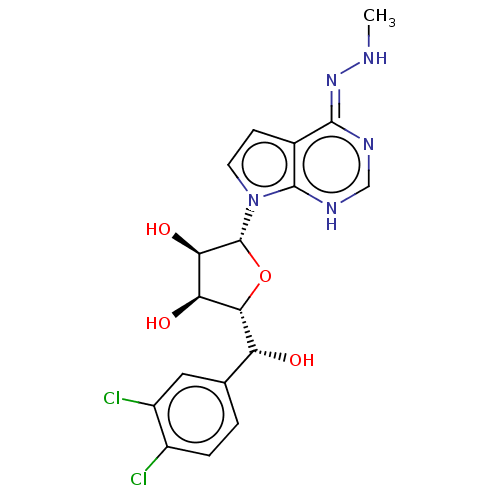
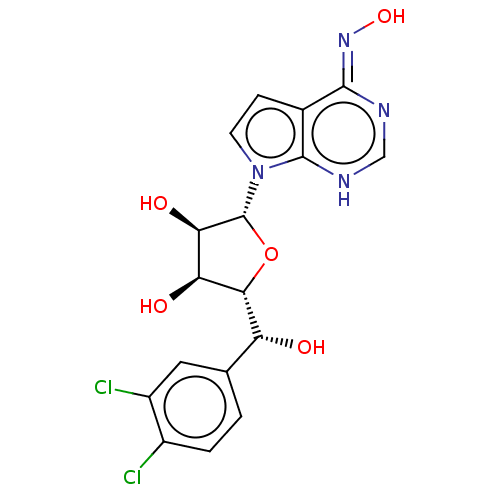
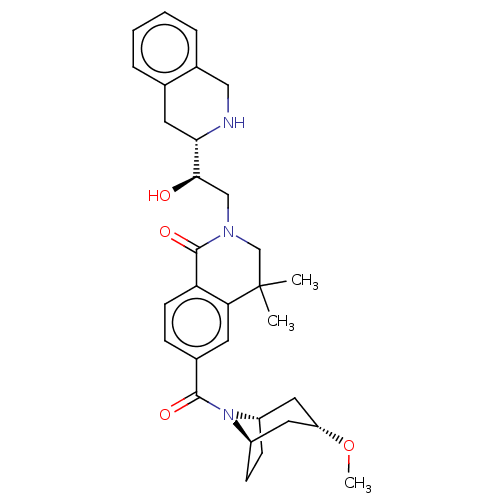
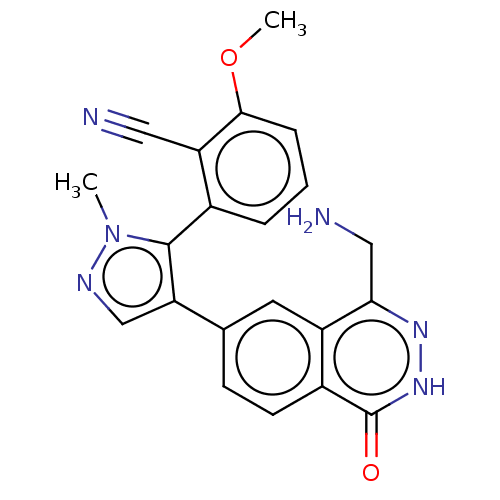
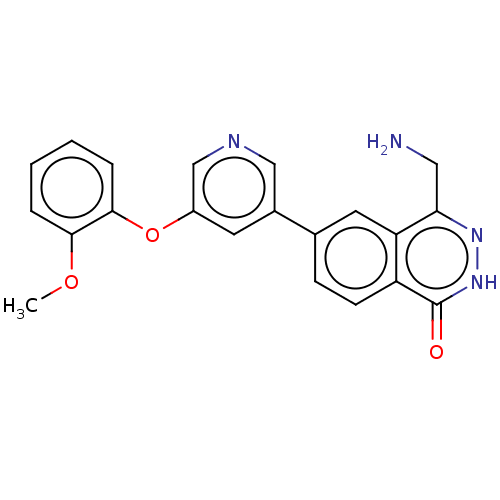
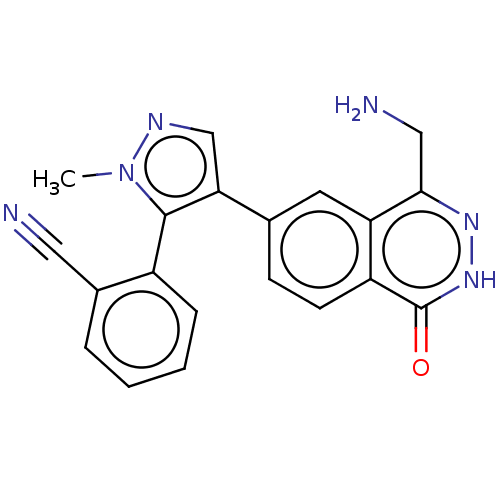
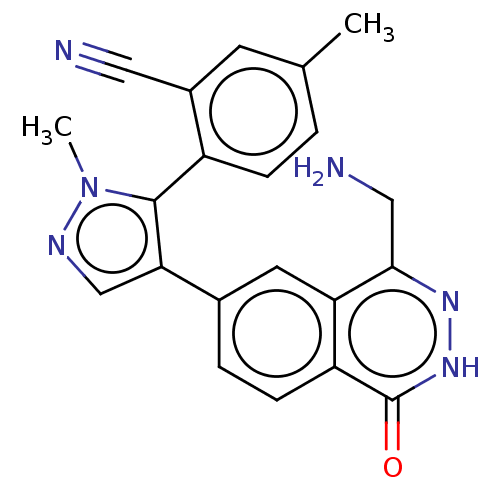
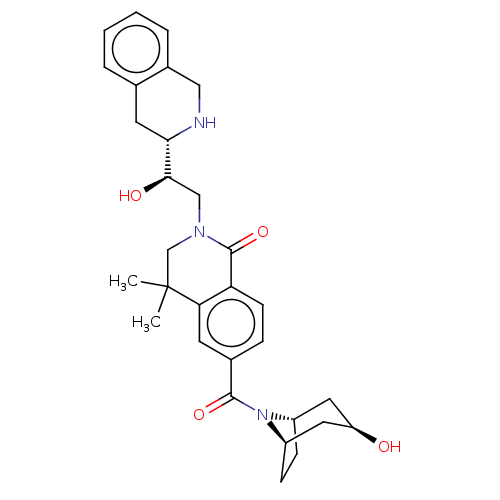
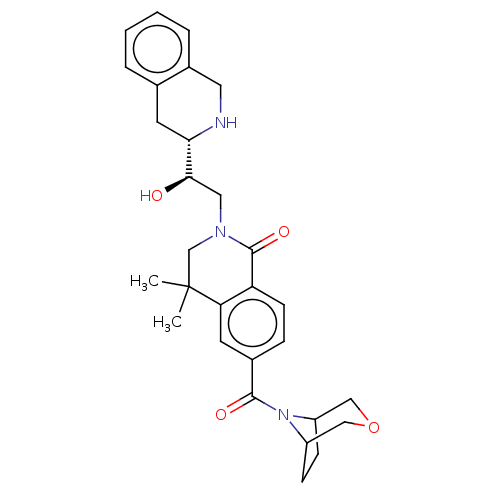
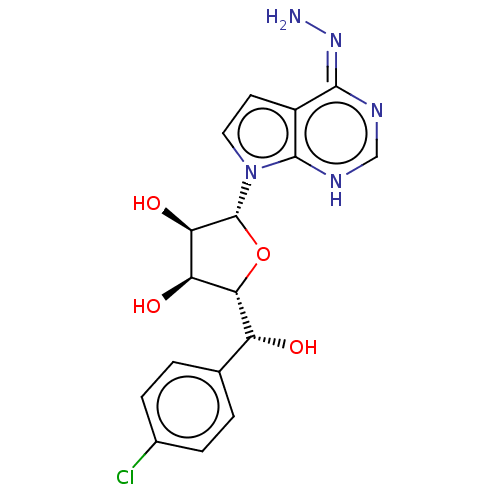
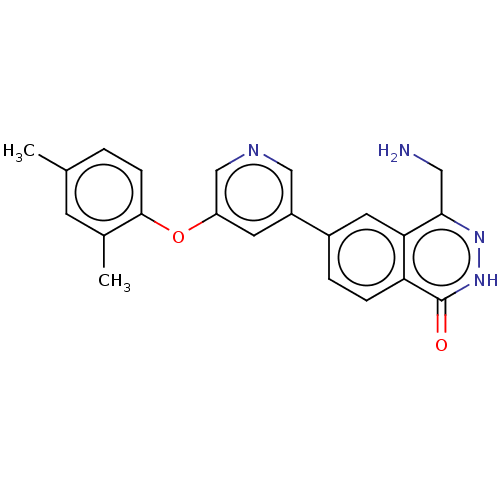
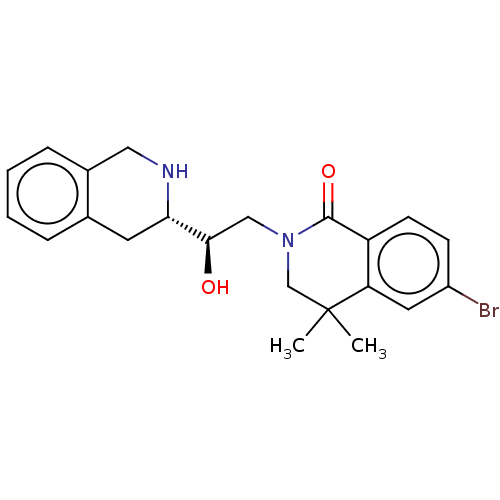
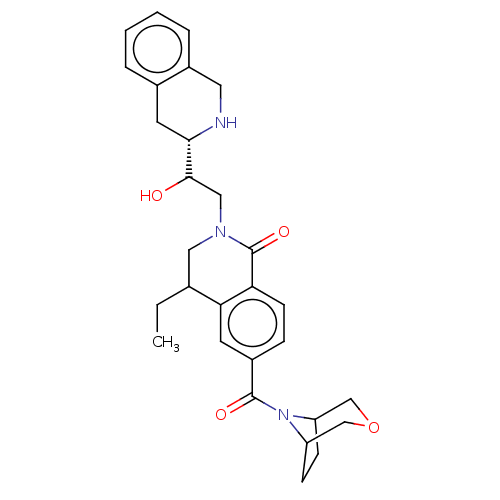
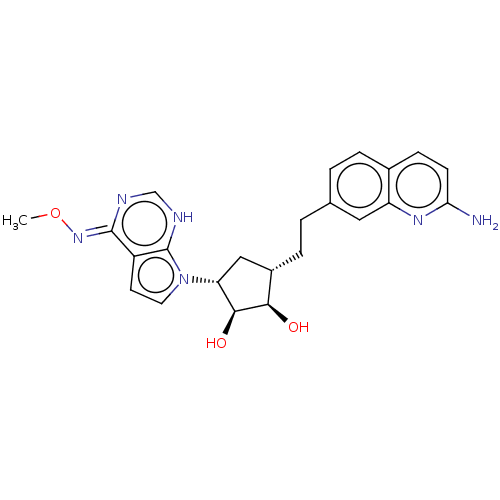
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.130nMAssay Description:Inhibition of full-length human N-terminal FLAG-tagged PRMT5/full length N-terminal His6-tagged MEP50 (unknown origin) expressed in baculovirus infec...More data for this Ligand-Target Pair
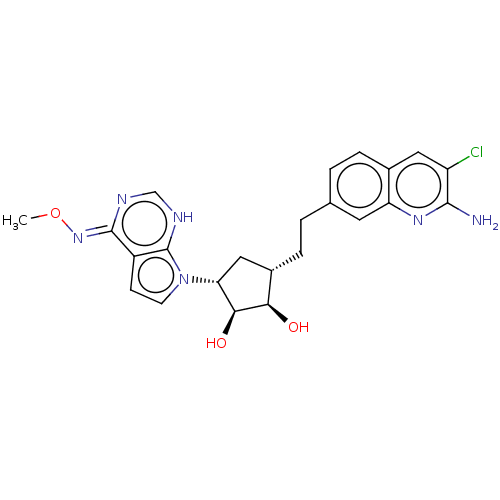
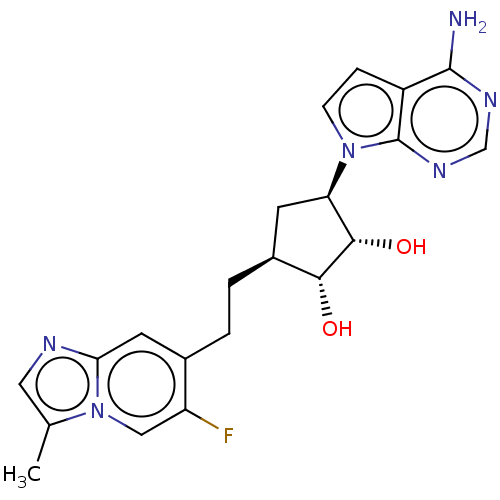
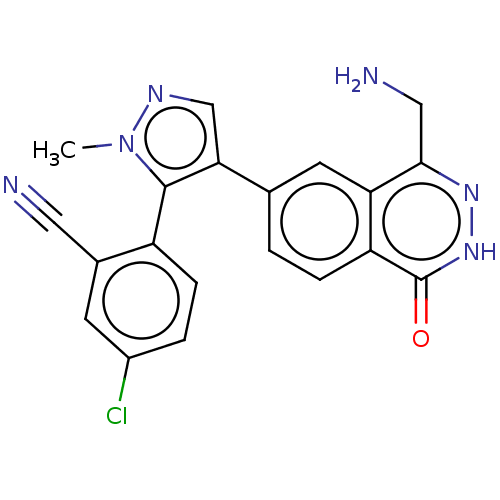
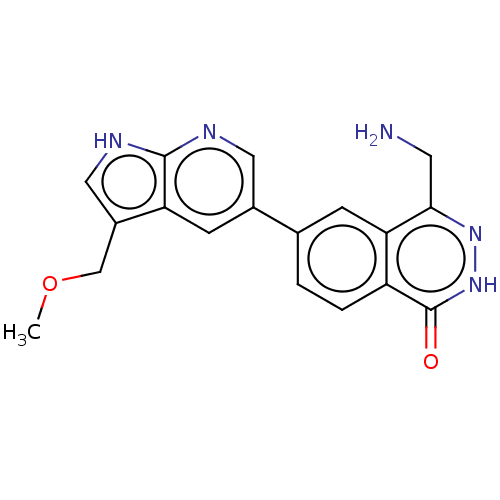
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.160nMAssay Description:Inhibition of full-length human N-terminal FLAG-tagged PRMT5/full length N-terminal His6-tagged MEP50 (unknown origin) expressed in baculovirus infec...More data for this Ligand-Target Pair
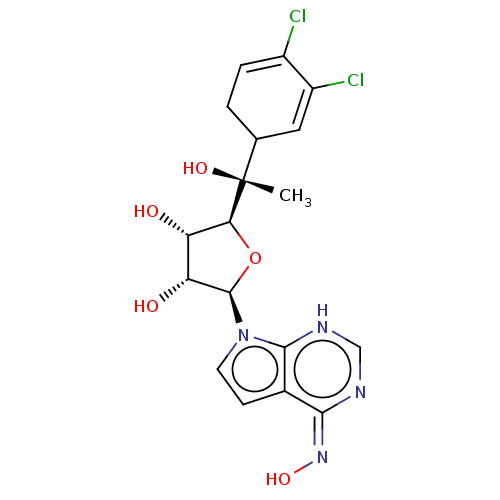
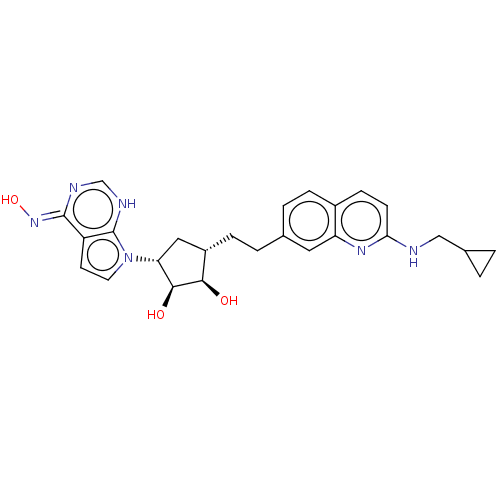
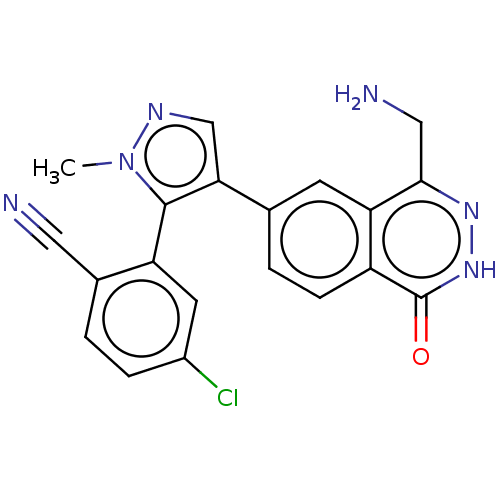
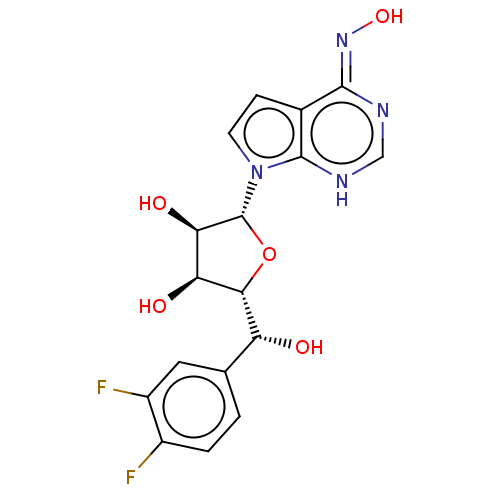
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of full-length human N-terminal FLAG-tagged PRMT5/full length N-terminal His6-tagged MEP50 (unknown origin) expressed in baculovirus infec...More data for this Ligand-Target Pair
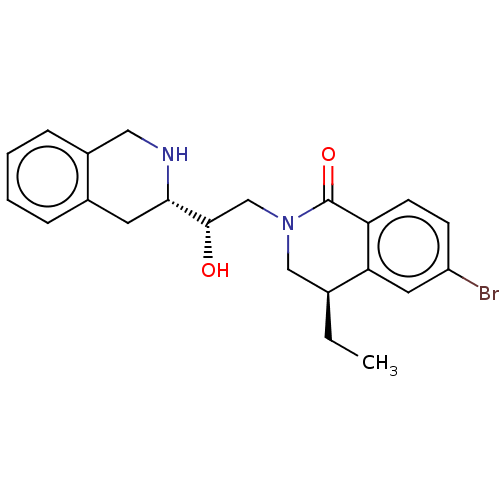
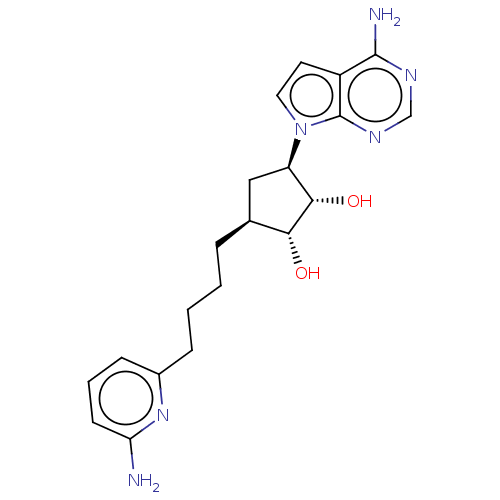
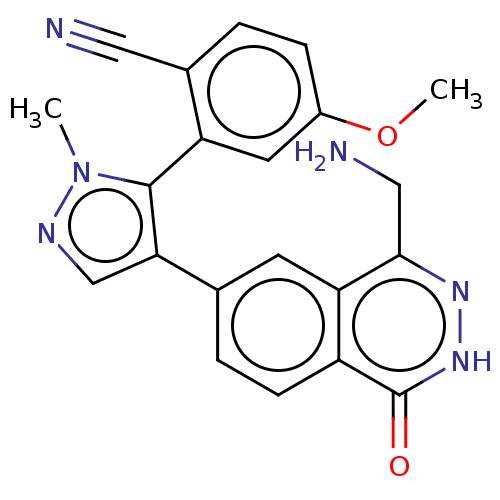
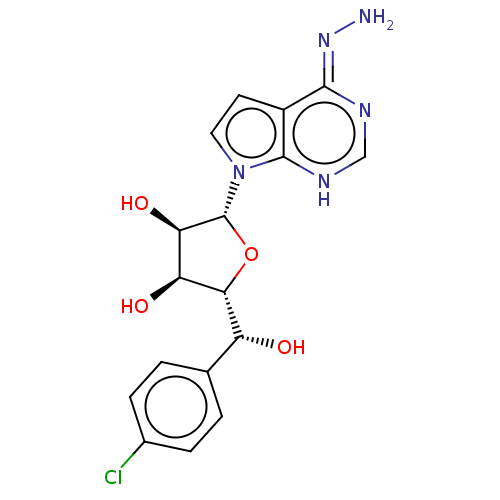
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Compounds were solubilized, and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Compounds were solubilized, and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.210nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.240nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.25nMAssay Description:Inhibition of full-length human N-terminal FLAG-tagged PRMT5/full length N-terminal His6-tagged MEP50 (unknown origin) expressed in baculovirus infec...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.290nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Compounds were solubilized, and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.310nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.320nMAssay Description:Inhibition of full-length human N-terminal FLAG-tagged PRMT5/full length N-terminal His6-tagged MEP50 (unknown origin) expressed in baculovirus infec...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.330nMAssay Description:Compounds were solubilized, and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.350nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.360nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of full-length human N-terminal FLAG-tagged PRMT5/full length N-terminal His6-tagged MEP50 (unknown origin) expressed in baculovirus infec...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Compounds were solubilized, and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.440nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.470nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5,...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5,...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.520nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.530nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Compounds were solubilized, and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Compounds were solubilized, and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of PRMT5 (unknown origin)/MEP50 (unknown origin) using histone H2 as substrate preincubated for 15 to 20 mins followed by S-[methyl-3H]ade...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.610nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.630nMAssay Description:In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.640nMAssay Description:Compounds were solubilized, and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5...More data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Prelude Therapeutics
Curated by ChEMBL
Prelude Therapeutics
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:HotSpot Assay. Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tr...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)