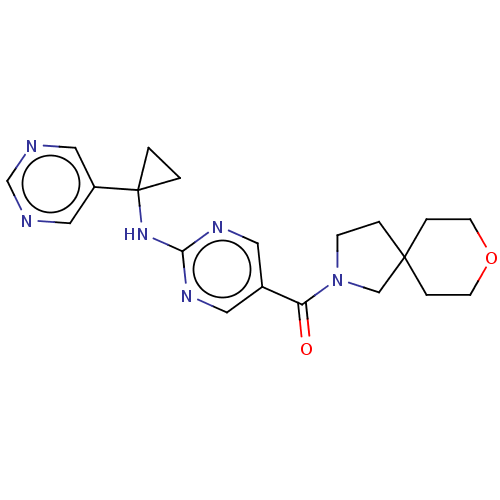
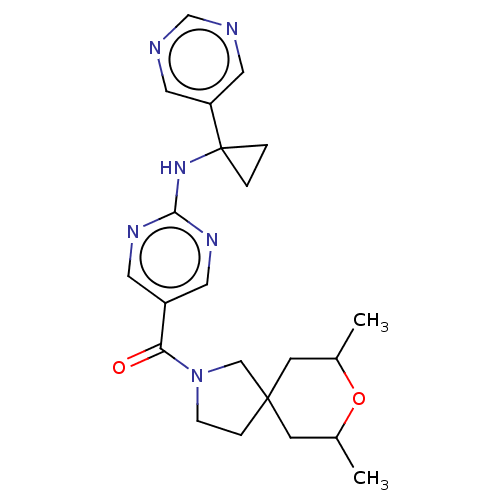
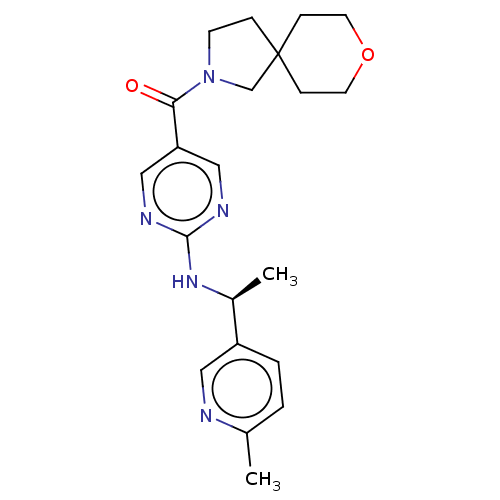
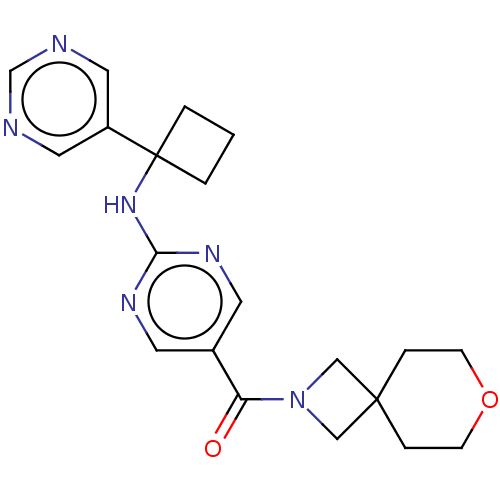
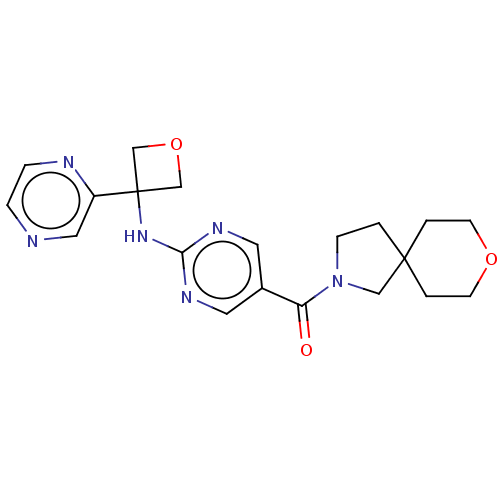
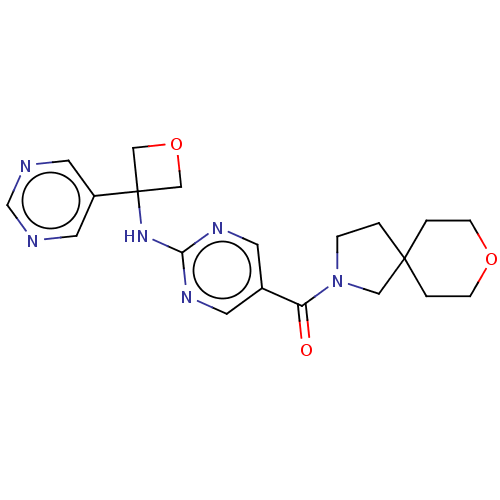
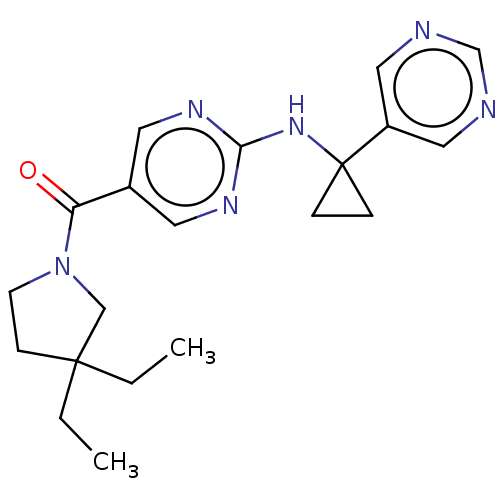
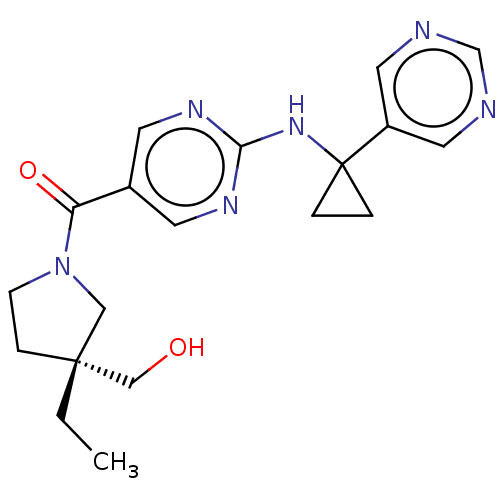
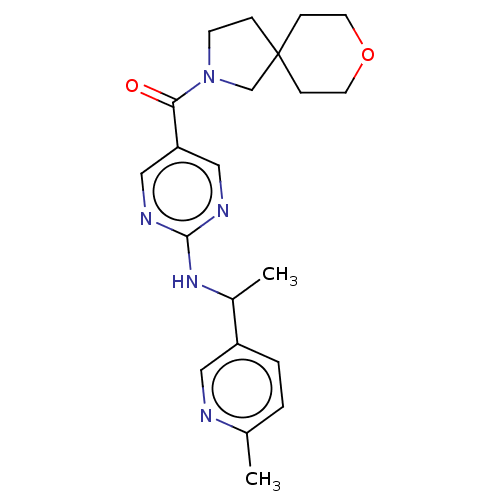
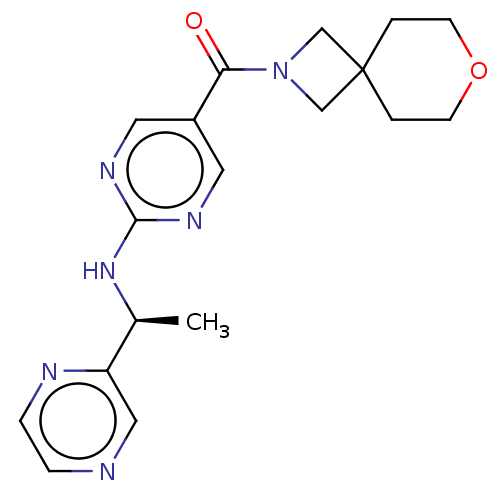
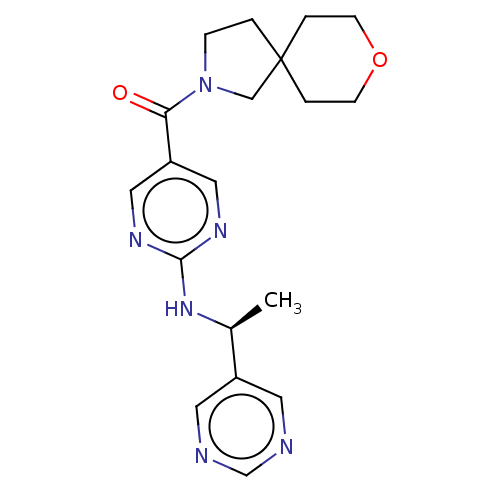
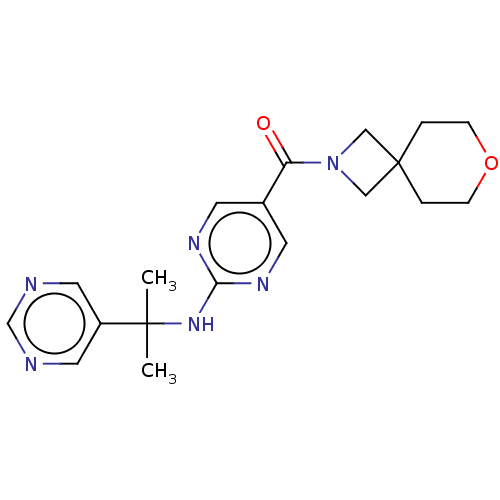
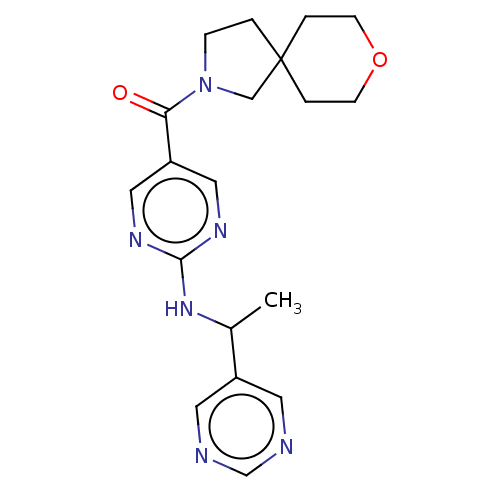
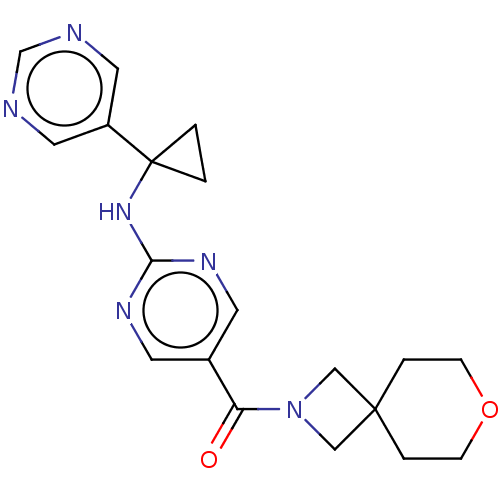
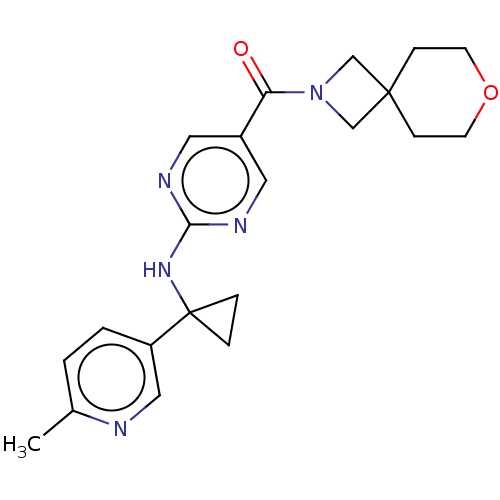
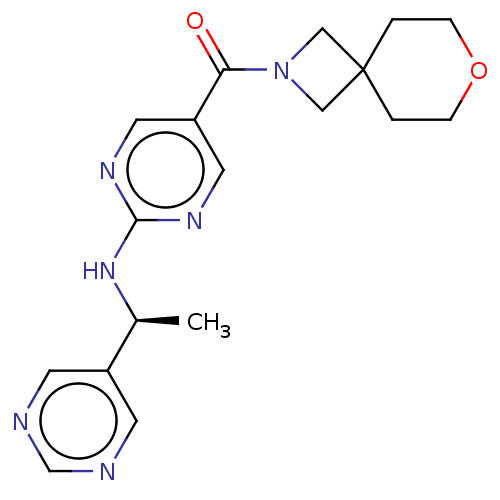
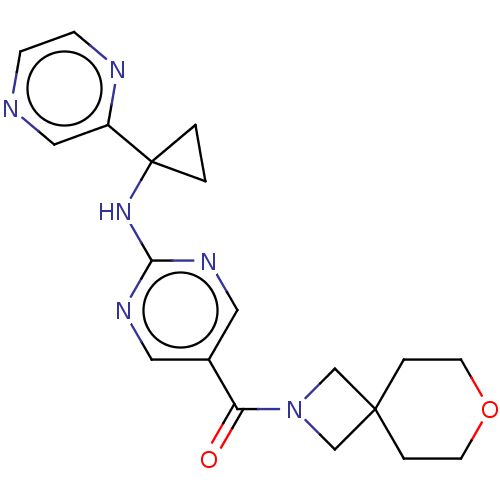
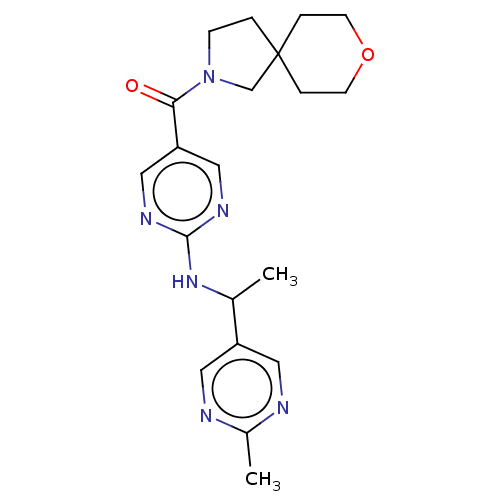
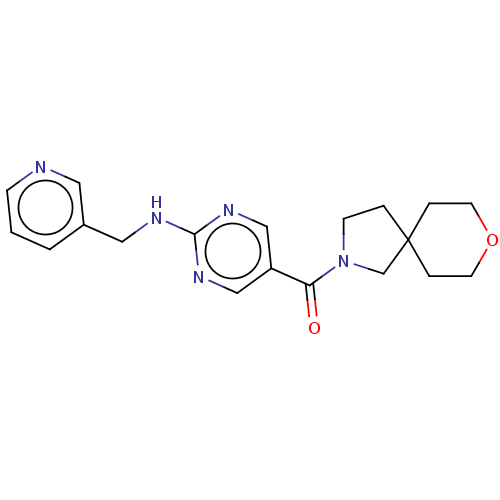
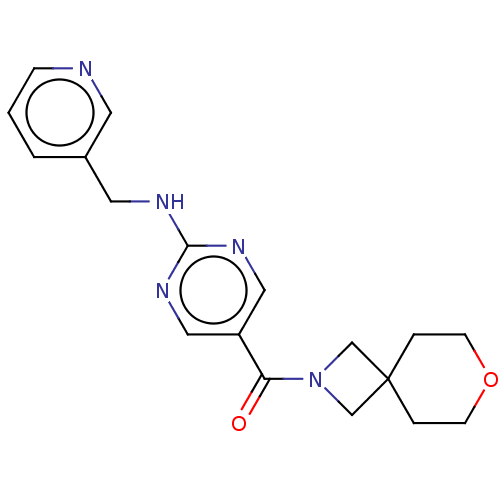
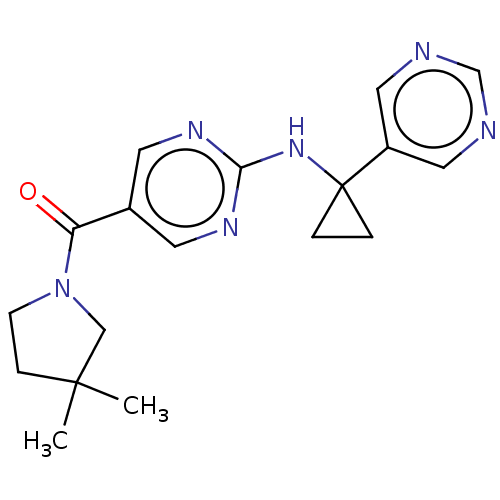
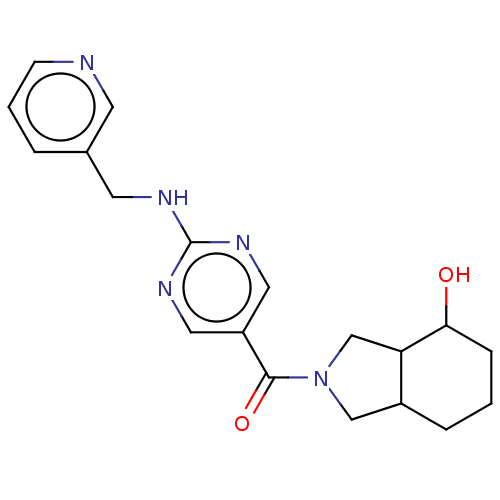
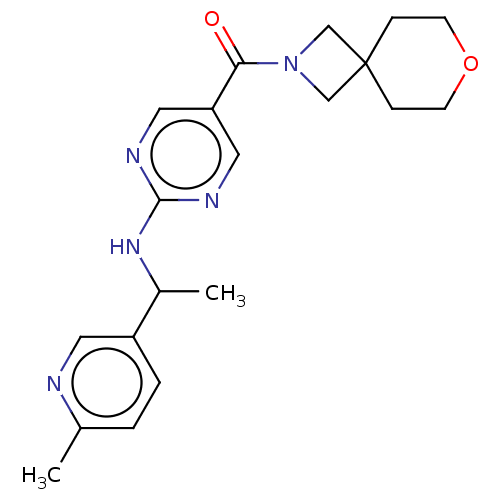
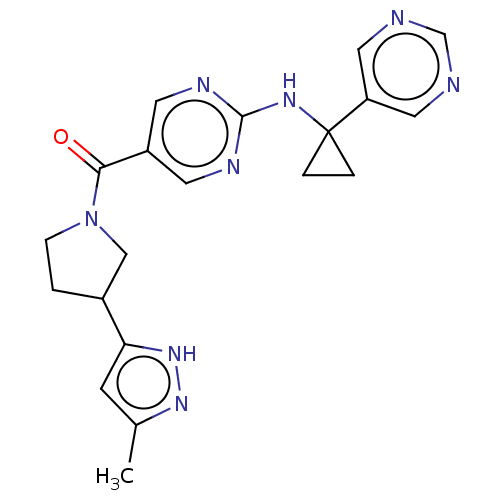
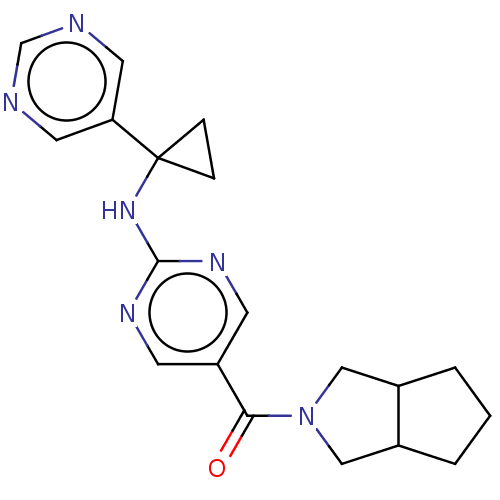
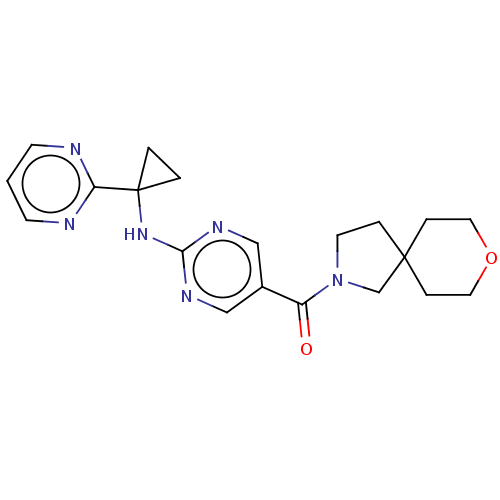
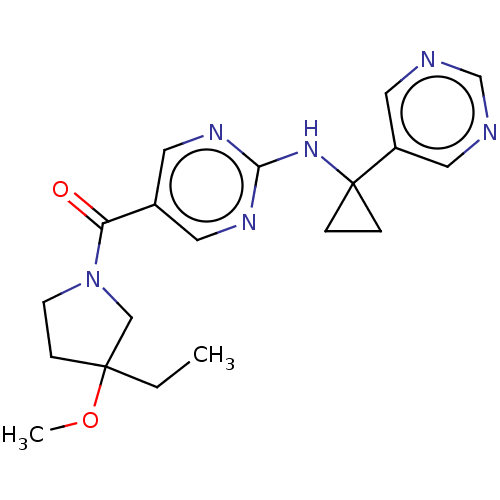
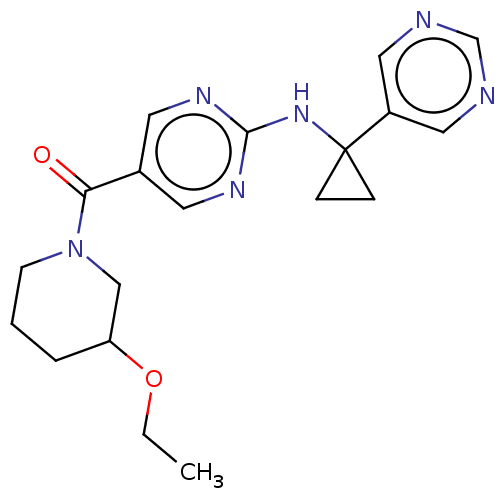
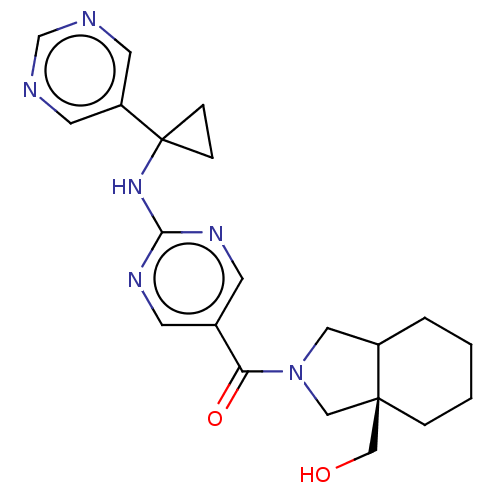
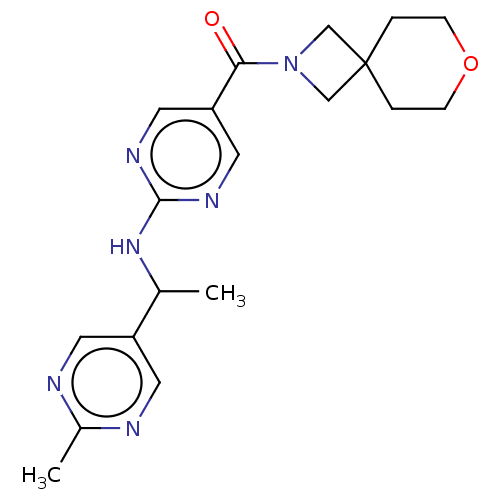
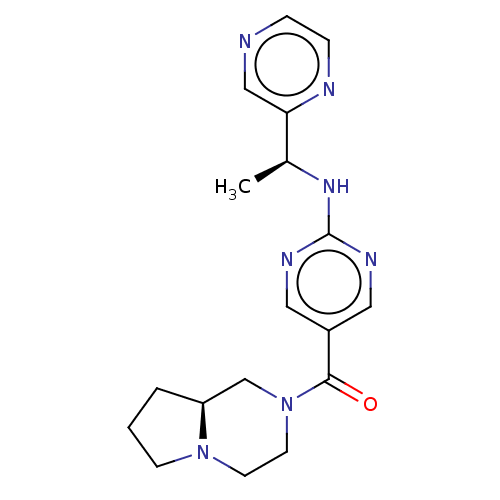
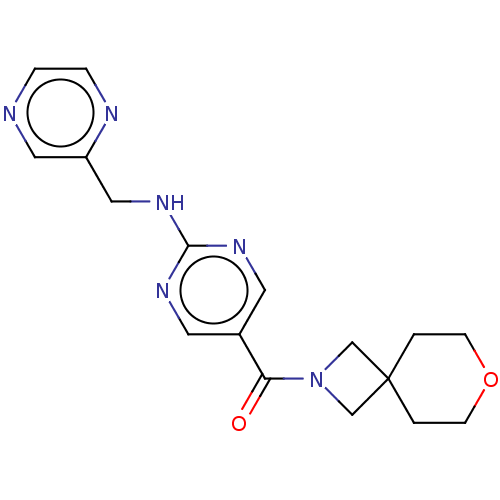
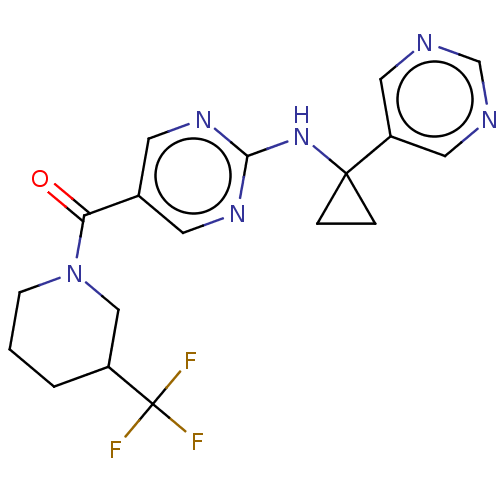
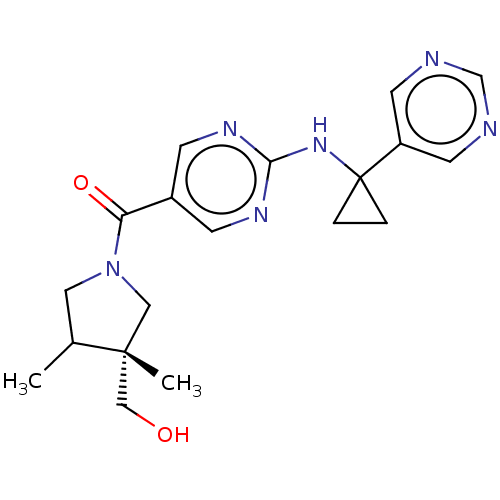
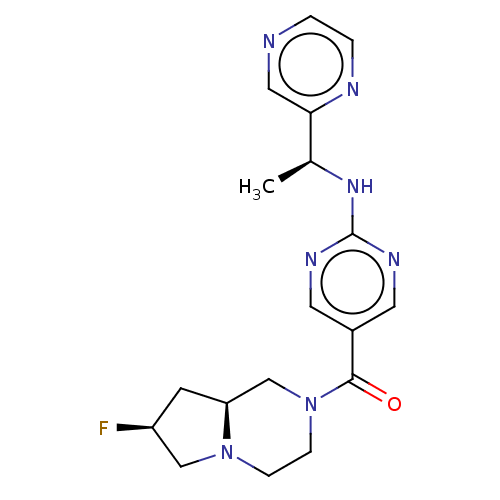
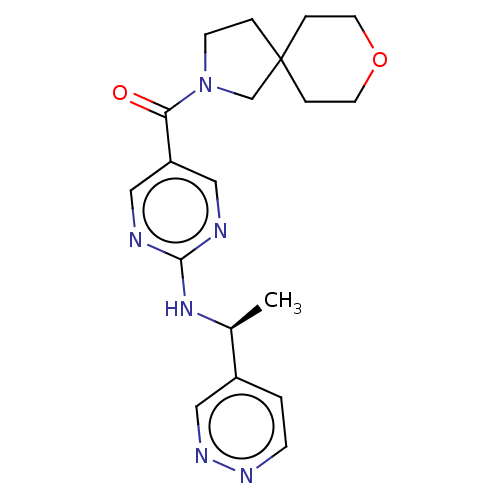
Affinity DataIC50: <0.0410nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0430nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0620nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0820nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: <0.0940nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.116nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.196nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.240nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.242nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.277nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.283nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.283nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.293nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.349nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.504nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.504nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.551nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.582nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.629nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.650nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.677nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.743nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.745nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.867nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.875nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.911nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 0.955nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.11nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.29nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.31nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.35nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.45nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.57nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.69nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.77nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.82nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.85nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 2.18nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 2.21nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 2.45nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 2.51nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 2.84nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 3.04nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 3.38nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 3.62nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 3.92nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 3.95nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair
Affinity DataIC50: 4.29nMAssay Description:The test inhibitors were solubilized in DMSO to a stock concentration of 30 mM. On the day of the assay, dose response plates were prepared by diluti...More data for this Ligand-Target Pair

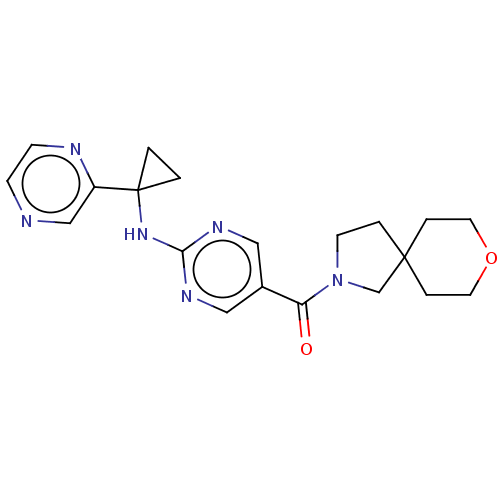
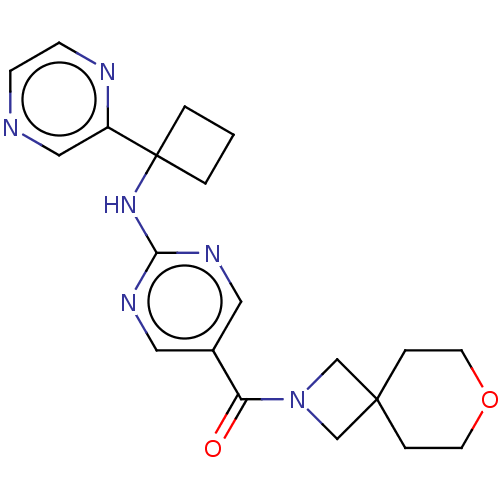
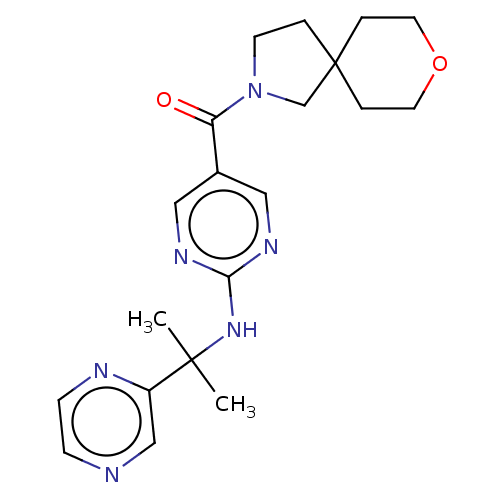
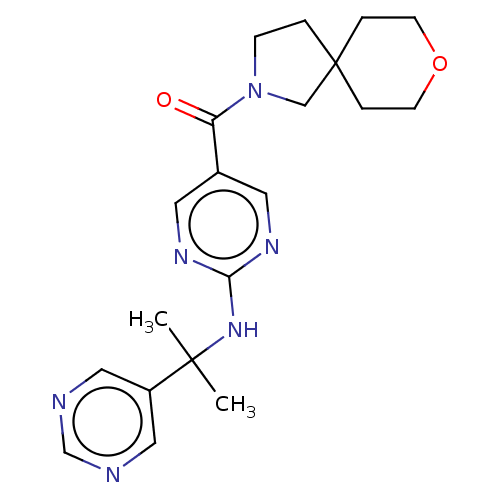
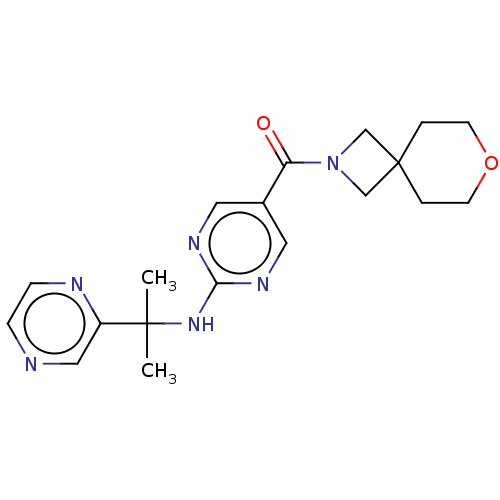
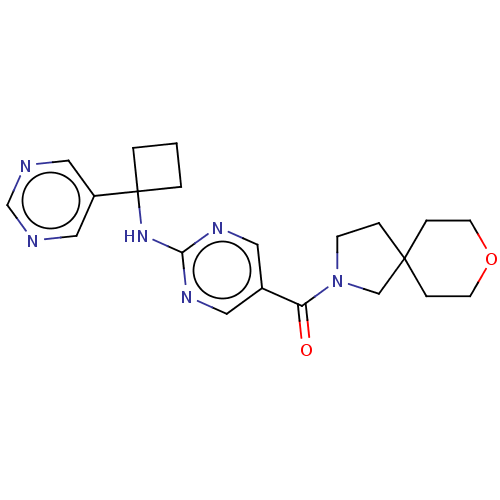
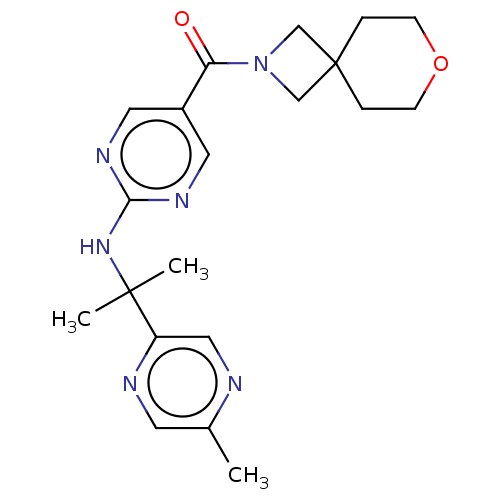
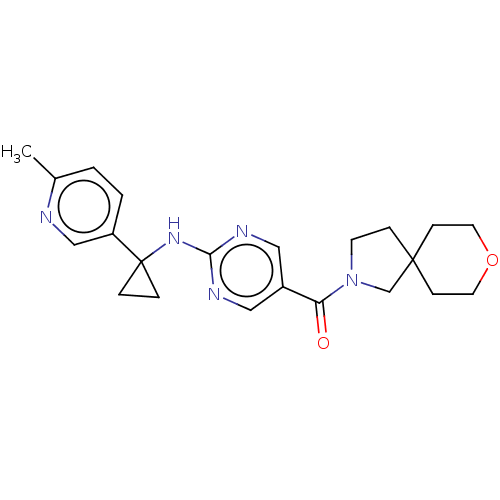
 3D Structure (crystal)
3D Structure (crystal)