Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 2A
Ligand
BDBM35240
Substrate
BDBM21395
Meas. Tech.
Radioligand Binding Assay (Ki) and Norepinephrine Uptake Assay (IC50)
Citation
 Heffernan, GD; Coghlan, RD; Manas, ES; McDevitt, RE; Li, Y; Mahaney, PE; Robichaud, AJ; Huselton, C; Alfinito, P; Bray, JA; Cosmi, SA; Johnston, GH; Kenney, T; Koury, E; Winneker, RC; Deecher, DC; Trybulski, EJ Dual acting norepinephrine reuptake inhibitors and 5-HT(2A) receptor antagonists: Identification, synthesis and activity of novel 4-aminoethyl-3-(phenylsulfonyl)-1H-indoles. Bioorg Med Chem 17:7802-15 (2009) [PubMed] Article
Heffernan, GD; Coghlan, RD; Manas, ES; McDevitt, RE; Li, Y; Mahaney, PE; Robichaud, AJ; Huselton, C; Alfinito, P; Bray, JA; Cosmi, SA; Johnston, GH; Kenney, T; Koury, E; Winneker, RC; Deecher, DC; Trybulski, EJ Dual acting norepinephrine reuptake inhibitors and 5-HT(2A) receptor antagonists: Identification, synthesis and activity of novel 4-aminoethyl-3-(phenylsulfonyl)-1H-indoles. Bioorg Med Chem 17:7802-15 (2009) [PubMed] Article More Info.:
Target
Name:
5-hydroxytryptamine receptor 2A
Synonyms:
5-HT-2 | 5-HT-2A | 5-HT2A | 5-hydroxytryptamine receptor 2A (5-HT-2A) | 5-hydroxytryptamine receptor 2A (5HT-2A) | 5-hydroxytryptamine receptor 2A (5HT2A) | 5HT2A_HUMAN | HTR2 | HTR2A | Serotonin receptor 2A
Type:
undefined
Mol. Mass.:
52607.65
Organism:
Homo sapiens (Human)
Description:
P28223
Residue:
471
Sequence:
MDILCEENTSLSSTTNSLMQLNDDTRLYSNDFNSGEANTSDAFNWTVDSENRTNLSCEGCLSPSCLSLLHLQEKNWSALLTAVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIADMLLGFLVMPVSMLTILYGYRWPLPSKLCAVWIYLDVLFSTASIMHLCAISLDRYVAIQNPIHHSRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSFVSFFIPLTIMVITYFLTIKSLQKEATLCVSDLGTRAKLASFSFLPQSSLSSEKLFQRSIHREPGSYTGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNEDVIGALLNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENKKPLQLILVNTIPALAYKSSQLQMGQKKNSKQDAKTTDNDCSMVALGKQHSEEASKDNSDGVNEKVSCV
Inhibitor
Name:
BDBM35240
Synonyms:
3-(phenylsulfonyl)-1H-indole, 18m
Type:
Small organic molecule
Emp. Form.:
C16H16N2O2S
Mol. Mass.:
300.375
SMILES:
NCCc1cccc2[nH]cc(c12)S(=O)(=O)c1ccccc1
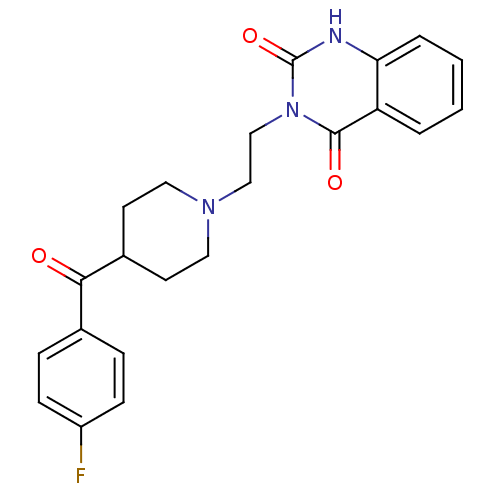
Substrate
Name:
BDBM21395
Synonyms:
3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H,3H)-quinazolinedione | 3-(2-{4-[(4-fluorophenyl)carbonyl]piperidin-1-yl}ethyl)-1,2,3,4-tetrahydroquinazoline-2,4-dione | CHEMBL1628637 | CHEMBL51 | Ketanserin | R-41,468 | R-41-468 | R41,468 | [3H]-Ketanserin
Type:
radiolabeled ligand
Emp. Form.:
C22H22FN3O3
Mol. Mass.:
395.4268
SMILES:
Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1