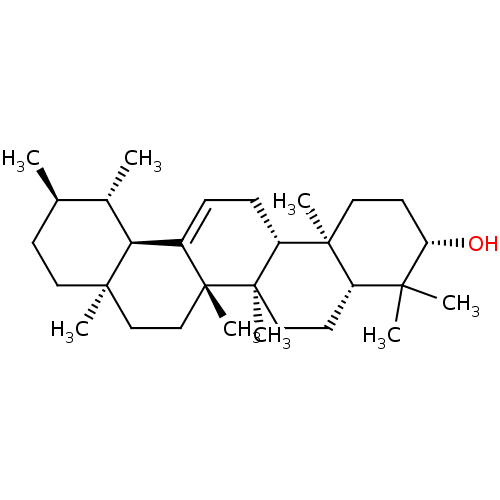
BDBM50241956 (3S,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-4,4,6a,6b,8a,11,12,14b-octamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-ol::CHEMBL455357::US11660306, Example amyrin::alpha-amyrin::urs-12-en-3beta-ol
SMILES C[C@@H]1CC[C@]2(C)CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C
InChI Key InChIKey=FSLPMRQHCOLESF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50241956
Found 8 hits for monomerid = 50241956
TargetTyrosine-protein phosphatase non-receptor type 1 [1-405](Human)
Cold Spring Harbor Laboratory
US Patent
Cold Spring Harbor Laboratory
US Patent
Affinity DataKi: 4.00E+3nMAssay Description:Glucose in tail blood was measured using a glucometer (One-Touch Basic; Lifescan, CA). For glucose tolerance tests (GTTs), mice were fasted for 10 ho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1 [1-321](Human)
Cold Spring Harbor Laboratory
US Patent
Cold Spring Harbor Laboratory
US Patent
Affinity DataKi: 6.00E+3nMAssay Description:Glucose in tail blood was measured using a glucometer (One-Touch Basic; Lifescan, CA). For glucose tolerance tests (GTTs), mice were fasted for 10 ho...More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at TGR5 expressed in CHO cells by CRE-driven luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.93E+4nMAssay Description:Inhibition of recombinant human GST-tagged PTP1B catalytic domain expressed in Escherichia coli BL21 using para-nitrophenyl phosphate as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant 5-LOX expressed in insect cells assessed as decrease in production of 5-HPETE and 5-HETE using arachidonic acid as su...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant 5-LOX expressed in insect cells assessed as decrease in production of 5-HPETE and 5-HETE using arachidonic acid as su...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:Inhibition of human DNA topoisomerase 2-mediated relaxation of supercoiled DNA by gel electrophoresisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:Inhibition of human DNA topoisomerase 1-mediated relaxation of supercoiled DNA by gel electrophoresisMore data for this Ligand-Target Pair