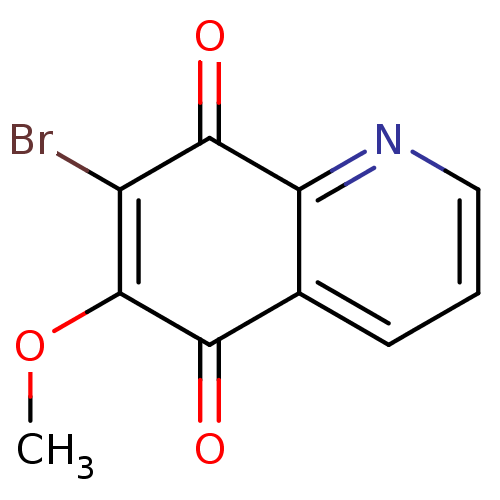
BDBM80075 7-bromanyl-6-methoxy-quinoline-5,8-dione::7-bromo-6-methoxy-quinoline-5,8-quinone::7-bromo-6-methoxyquinoline-5,8-dione::MLS002703741::SMR001570458::cid_265935
SMILES COC1=C(Br)C(=O)c2ncccc2C1=O
InChI Key InChIKey=VFJSDBUJXJBRRA-UHFFFAOYSA-N
Data 10 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 80075
Found 10 hits for monomerid = 80075
TargetBeta-galactosidase(Escherichia coli)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: >6.66E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetHexokinase HKDC1 [W721R](Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 3.56E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 6(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: >6.66E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA...More data for this Ligand-Target Pair
Affinity DataIC50: 3.53E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-1(Human)
The Scripps Research Institute-Florida
Curated by ChEMBL
The Scripps Research Institute-Florida
Curated by ChEMBL
Affinity DataIC50: 4.55E+3nMAssay Description:Inhibition of recombinant wild-type PAD1 (unknown origin) using N-alpha-Benzoyl-L-arginine amide as substrate preincubated for 15 mins followed by su...More data for this Ligand-Target Pair
Affinity DataIC50: >8.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-4(Human)
The Scripps Research Institute-Florida
Curated by ChEMBL
The Scripps Research Institute-Florida
Curated by ChEMBL
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of recombinant wild-type PAD4 (unknown origin) using N-alpha-Benzoyl-L-arginine ethyl ester as substrate preincubated for 15 mins followed...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-3(Human)
The Scripps Research Institute-Florida
Curated by ChEMBL
The Scripps Research Institute-Florida
Curated by ChEMBL
Affinity DataIC50: 4.40E+3nMAssay Description:Inhibition of recombinant wild-type PAD3 (unknown origin) using N-alpha-Benzoyl-L-arginine amide as substrate preincubated for 15 mins followed by su...More data for this Ligand-Target Pair
TargetProtein-arginine deiminase type-2(Human)
The Scripps Research Institute-Florida
Curated by ChEMBL
The Scripps Research Institute-Florida
Curated by ChEMBL
Affinity DataIC50: 6.32E+3nMAssay Description:Inhibition of recombinant wild-type PAD2 (unknown origin) using N-alpha-Benzoyl-L-arginine ethyl ester as substrate preincubated for 15 mins followed...More data for this Ligand-Target Pair
TargetHexokinase HKDC1 [W721R](Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 4.89E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair