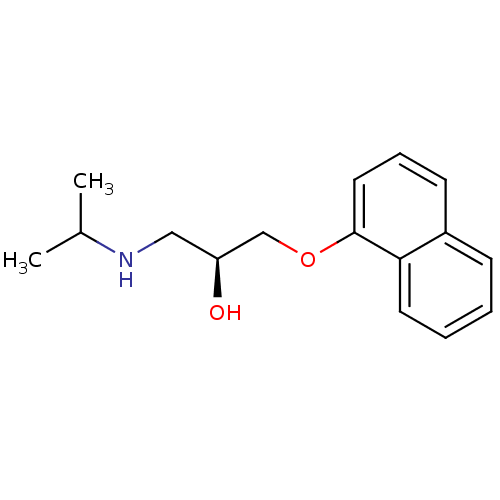
BDBM50246936 (-)-(S)-Propranolol::1-(ISOPROPYLAMINO)-3-(1-NAPHTHYLOXY)-2-PROPANOL::CHEMBL452861::PROPRANOLOL::S-(-)-propanolol::[2-Hydroxy-3-(naphthalen-1-yloxy)-propyl]-isopropyl-ammonium((-)-propranolol)
SMILES CC(C)NC[C@H](O)COc1cccc2ccccc12
InChI Key InChIKey=AQHHHDLHHXJYJD-AWEZNQCLSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 22 hits for monomerid = 50246936
Found 22 hits for monomerid = 50246936
Affinity DataKi: 17nMAssay Description:Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat striatal membrane homogenate using [3H]5-HT as the radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Binding affinity (Ki) to rat cortical membranes at 5-HT1B binding site by using [125 I] ICYP as a radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:Inhibitory activity against 5-hydroxytryptamine 1A receptor of rat hippocampal tissue using [3H]OH-DPAT as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 115nMAssay Description:Evaluated for the binding affinity to hippocampus striatal membranes at 5-hydroxytryptamine 1A receptor binding site by using [3H]-8-OH- DPAT as a ra...More data for this Ligand-Target Pair
Affinity DataKi: 300nMAssay Description:Evaluated for binding affinity towards rat cortical membranes at 5-hydroxytryptamine 1 receptor binding site by using [3H]-5-HT as a radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:Evaluated for the binding affinity to porcine choroid plexus at 5-hydroxytryptamine 2C receptor binding site by using [3H]-MES as a radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 3.50E+3nMAssay Description:Binding affinity to 5-hydroxytryptamine 2 receptor in rat frontal cortical membranes by [3H]- KET displacement.More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1B(Human)
Medical College of Virginia/Virginia Commonwealth University
Curated by ChEMBL
Medical College of Virginia/Virginia Commonwealth University
Curated by ChEMBL
Affinity DataKi: 4.10E+3nMAssay Description:Agonist activity to the human recombinant 5-hydroxytryptamine 1B receptorMore data for this Ligand-Target Pair
Affinity DataKi: 5.90E+3nMAssay Description:Binding affinity to rat cortical membranes at 5-hydroxytryptamine 2 (5-HT2) receptor using [3H]KET as a radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 8.10E+3nMAssay Description:Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.70E+5nMAssay Description:Concentration giving half of the maximal ATPase activity calculated for the high-affinity binding site of the CHO P-Glycoprotein (P-gp) in two-affini...More data for this Ligand-Target Pair
Affinity DataKd: 0.700nMAssay Description:Binding affinity against Beta adrenergic receptor in C6-2B astrocytoma cells, using [125I]-iodohydroxybenzylpindolol-benzylpindolol([125I]-HYP)More data for this Ligand-Target Pair
TargetSodium/bile acid cotransporter(Human)
Vanderbilt University School of Medicine
Curated by ChEMBL
Vanderbilt University School of Medicine
Curated by ChEMBL
Affinity DataIC50: 6.10E+3nMAssay Description:TP_TRANSPORTER: inhibition of Taurocholate uptake in NTCP-expressing HeLa cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 1.80nMAssay Description:Displacement of [125I]cyanopindolol from human recombinant adrenergic beta-1 receptor expressed in Rex16 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of binding of Batrachotoxinin [3H]BTX-B to high affinity sites on voltage dependent sodium channels in a vesicular preparation from guinea...More data for this Ligand-Target Pair
Affinity DataIC50: 5.74E+5nMAssay Description:Concentration required for 50% inhibition at binding site of human P-Glycoprotein (P-gp) in one-affinity modelMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group E member 1(Human)
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataEC50: 7.20E+4nMAssay Description:Agonist activity at in human TLX LBD expressed in human HEK293T cells coexpressing Gal4-VP 16 assessed as increase in reporter activity measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D2 expressed in Saccharomyces cerevisiaeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of MAMC O-dealkylation mediated by human Cytochrome P450 2D6 expressed in human lymphoblastoid cell lineMore data for this Ligand-Target Pair
Affinity DataIC50: 5.71E+5nMAssay Description:Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D1 expressed in Saccharomyces cerevisiaeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiaeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.72E+5nMAssay Description:Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D3 expressed in Saccharomyces cerevisiaeMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)