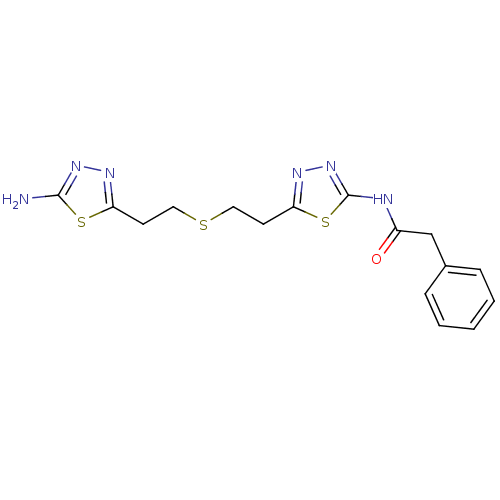
BDBM108460 CHEMBL2178393::US11191732, Example 1::US8604016, 17::US9938267, Cmpd ID 17
SMILES Nc1nnc(CCSCCc2nnc(NC(=O)Cc3ccccc3)s2)s1
InChI Key InChIKey=SWXIKLMDWGQGKU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 108460
Found 7 hits for monomerid = 108460
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Uncompetitive inhibition of human kidney glutaminase (124 to 669) assessed as reduction of glutamine hydrolysis by double-reciprocal plot analysisMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human kidney glutaminase (124 to 669) using L-[3H]-glutamine as substrate after 45 mins by GLS assayMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 2.70E+3nMAssay Description:A high throughput screening (HTS) assay and together with NCATS screened over 350,000 compounds in attempt to identify novel GLS inhibitor structures...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:No preinc (Column 4).More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human KGA preincubated for 15 to 30 mins followed by substrate addition and measured after 3 to 4 hrs by GDH-EZMTT reagent-based GDH co...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Compounds were assessed for their ability to inhibit the enzymatic activity of a recombinant form of Glutaminase 1 (GAC) using a biochemical assay th...More data for this Ligand-Target Pair
TargetGlutaminase liver isoform, mitochondrial(Mus musculus)
Johns Hopkins University
Curated by ChEMBL
Johns Hopkins University
Curated by ChEMBL
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of mouse GLS2 using glutamine as substrate by fluorescence-based GLS assayMore data for this Ligand-Target Pair