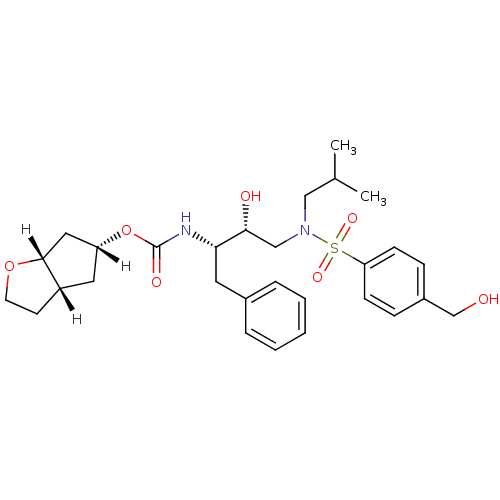
BDBM13925 (3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl N-[(2S,3R)-3-hydroxy-4-{[4-(hydroxymethyl)benzene](2-methylpropyl)sulfonamido}-1-phenylbutan-2-yl]carbamate::GRL-06579A::hexahydrocyclopenta[b]furanyl urethane analog 3
SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@@H]1C[C@@H]2CCO[C@@H]2C1)S(=O)(=O)c1ccc(CO)cc1
InChI Key InChIKey=VYBDPVQMILRSMK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 13925
Found 4 hits for monomerid = 13925
TargetProtease(Human immunodeficiency virus type 1)
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences
Curated by ChEMBL
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 0.00350nMAssay Description:Inhibition of HIV1 protease dimerization in MT2 cellsMore data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.00450nM ΔG°: -15.5kcal/molepH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus type 1)
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences
Curated by ChEMBL
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 0.00500nMAssay Description:Inhibition of HIV1 proteaseMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus type 1)
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences
Curated by ChEMBL
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of HIV1 proteaseMore data for this Ligand-Target Pair