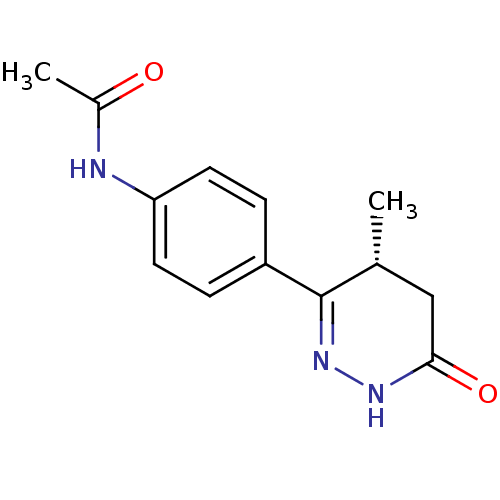
BDBM15298 N-{4-[(4R)-4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl]phenyl}acetamide::dihydropyridazinone 6a
SMILES C[C@@H]1CC(=O)NN=C1c1ccc(NC(C)=O)cc1
InChI Key InChIKey=GDZXNMWZXLDEKG-MRVPVSSYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 15298
Found 4 hits for monomerid = 15298
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 37nMpH: 7.5 T: 2°CAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant PDE3BMore data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of human N-terminal His-GST-tagged recombinant PDE3A (669-end residues) using fluorescent labelled cAMP as substrateMore data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A [388-1112](Homo sapiens (Human))
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 96nMAssay Description:PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t...More data for this Ligand-Target Pair