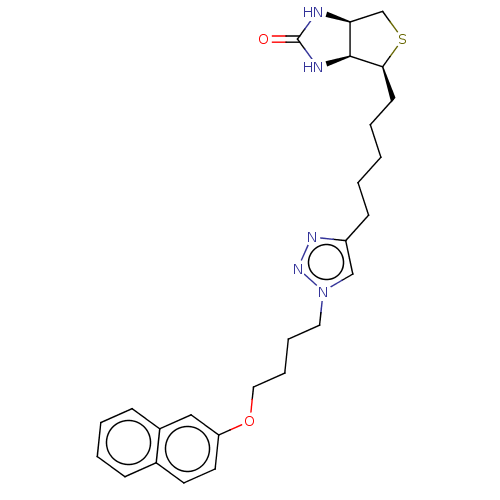
BDBM161469 US9108978, 6.07
SMILES O=C1N[C@H]2CS[C@@H](CCCCCc3cn(CCCCOc4ccc5ccccc5c4)nn3)[C@H]2N1
InChI Key InChIKey=PLGWUMURZLHGNO-SDHOMARFSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 161469
Found 3 hits for monomerid = 161469
Affinity DataKi: 1.17E+3nM ΔG°: -8.41kcal/mole IC50: 7.00E+3nMpH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
Affinity DataKi: >3.00E+4nM ΔG°: >-6.41kcal/molepH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
Affinity DataKi: >3.00E+4nM ΔG°: >-6.41kcal/molepH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair