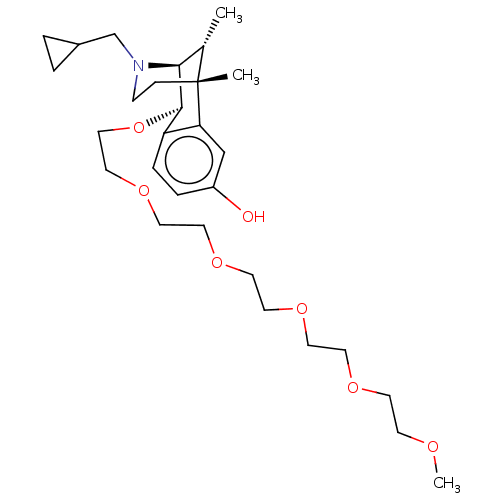
BDBM168151 US10081602, Example 25::US9688638, 25
SMILES COCCOCCOCCOCCOCCO[C@H]1[C@@H]2[C@H](C)[C@@](C)(CCN2CC2CC2)c2cc(O)ccc12
InChI Key InChIKey=COCOGGUKZAWHKM-KITINDFRSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 168151
Found 8 hits for monomerid = 168151
Affinity DataKi: 955nMpH: 7.5Assay Description:The binding affinities of certain compounds of the present invention were evaluated using radioligand binding assays in membranes prepared from CHO-K...More data for this Ligand-Target Pair
Affinity DataKi: 955nM ΔG°: -8.21kcal/molepH: 7.5 T: 2°CAssay Description:Competition binding experiments were conducted by incubating membrane protein to equilibrium in triplicate in the presence of a fixed concentration o...More data for this Ligand-Target Pair
Affinity DataKi: 3.92E+3nM ΔG°: -7.37kcal/molepH: 7.5 T: 2°CAssay Description:Competition binding experiments were conducted by incubating membrane protein to equilibrium in triplicate in the presence of a fixed concentration o...More data for this Ligand-Target Pair
Affinity DataKi: 3.92E+3nMpH: 7.5Assay Description:The binding affinities of certain compounds of the present invention were evaluated using radioligand binding assays in membranes prepared from CHO-K...More data for this Ligand-Target Pair
Affinity DataEC50: 195nMAssay Description:Inhibition of cAMP accumulation by select compounds was measured in forskolin-stimulated CHO-K1 cells stably expressing KOR. CHO-K1 cells stably expr...More data for this Ligand-Target Pair
Affinity DataEC50: 1.21E+3nMAssay Description:Inhibition of cAMP accumulation by select compounds was measured in forskolin-stimulated CHO-K1 cells stably expressing KOR. CHO-K1 cells stably expr...More data for this Ligand-Target Pair
Affinity DataEC50: 195nMAssay Description:Inhibition of cAMP accumulation by select compounds was measured in forskolin-stimulated CHO-K1 cells stably expressing KOR. CHO-K1 cells stably expr...More data for this Ligand-Target Pair
Affinity DataEC50: 1.21E+3nMAssay Description:Inhibition of cAMP accumulation by select compounds was measured in forskolin-stimulated CHO-K1 cells stably expressing KOR. CHO-K1 cells stably expr...More data for this Ligand-Target Pair