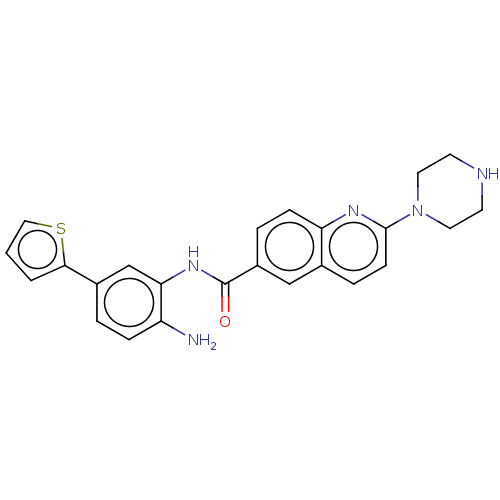
BDBM182757 US9145412, Table 1, Compound 23
SMILES Nc1ccc(cc1NC(=O)c1ccc2nc(ccc2c1)N1CCNCC1)-c1cccs1
InChI Key InChIKey=VURDNNVAYZDGDK-UHFFFAOYSA-N
Data 12 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 182757
Found 12 hits for monomerid = 182757
Affinity DataIC50: 4nMpH: 7.4Assay Description:Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMpH: 7.4Assay Description:Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were...More data for this Ligand-Target Pair
Affinity DataIC50: 114nMpH: 7.4Assay Description:Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were...More data for this Ligand-Target Pair
TargetHistone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)(Homo sapiens (Human))
University Of East Anglia
Curated by ChEMBL
University Of East Anglia
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of His-tagged GST-fused human HDAC3/NcoR2 expressed in sf9 cells using acetyl-lysine tripeptide as substrate preincubated for 10 mins foll...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human HDAC4 expressed in baculovirus infected sf9 cells using acetyl-lysine tripeptide as substrate preincubated for 10 mins followed b...More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inhibition of human HDAC2 expressed in Sf9 cells using acetyl-lysine tripeptide as substrate preincubated for 10 mins followed by substrate addition ...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of HDAC6 (unknown origin) using acetyl-lysine tripeptide as substrate preincubated for 10 mins followed by substrate addition and shaken f...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human N-terminal GST-tagged HDAC7 (518 to end residues) expressed in baculovirus infected sf9 cells using acetyl-lysine tripeptide as s...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of recombinant human C-terminal His-tagged HDAC8 expressed in baculovirus infected sf9 cells using acetyl-lysine tripeptide as substrate p...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of C-terminal His-tagged human HDAC9 (604 to 1066 residues) expressed in baculovirus infected sf9 cells using acetyl-lysine tripeptide as ...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of HDAC1 (unknown origin) using acetyl-lysine tripeptide as substrate preincubated for 10 mins followed by substrate addition and shaken f...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human HDAC5 using acetyl-lysine tripeptide as substrate preincubated for 10 mins followed by substrate addition and shaken for 5 secs a...More data for this Ligand-Target Pair