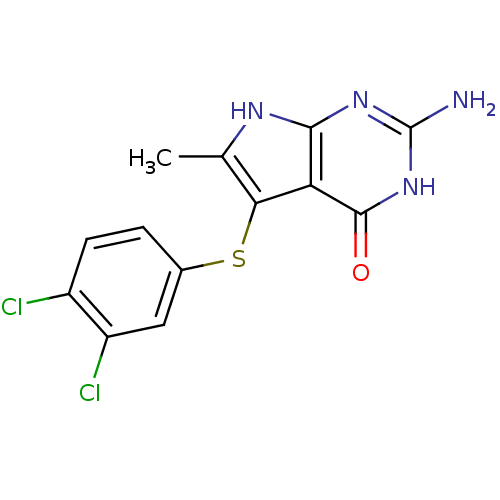
BDBM18808 2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-3H,4H,7H-pyrrolo[2,3-d]pyrimidin-4-one::CHEMBL400485::Pyrrolo[2,3-d]pyrimidine analogue, 3
SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(Cl)c(Cl)c1
InChI Key InChIKey=WWENIERBTIAOJG-UHFFFAOYSA-N
Data 15 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 18808
Found 15 hits for monomerid = 18808
Affinity DataIC50: 1.30E+4nMpH: 7.4 T: 2°CAssay Description:TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi...More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition of human thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMpH: 7.4 T: 2°CAssay Description:TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi...More data for this Ligand-Target Pair
Affinity DataIC50: 150nMpH: 7.4 T: 2°CAssay Description:TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi...More data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+4nMAssay Description:Inhibition of Escherichia coli thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:The inhibitory concentration of compound was evaluated on Pneumocystis carini Thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:The inhibitory concentration of compound was evaluated on Streptococcus faecium Thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:The inhibitory concentration of compound against Dihydrofolate reductases on Pneumocystis cariniMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:The inhibitory concentration of compound against Dihydrofolate reductases on rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+4nMAssay Description:The inhibitory concentration of compound was evaluated on Lactobacillus casei Thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:The inhibitory concentration of compound was evaluated on Human Thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+4nMAssay Description:The inhibitory concentration of compound was evaluated on Escherichia coli Thymidylate synthaseMore data for this Ligand-Target Pair
TargetBifunctional dihydrofolate reductase-thymidylate synthase(Toxoplasma gondii)
Duquesne University
Curated by ChEMBL
Duquesne University
Curated by ChEMBL
Affinity DataIC50: 1.17E+4nMAssay Description:The inhibitory concentration of compound against Dihydrofolate reductases on Toxoplasma gondiiMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibitory concentration against human thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: >6.20E+4nMAssay Description:DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy...More data for this Ligand-Target Pair