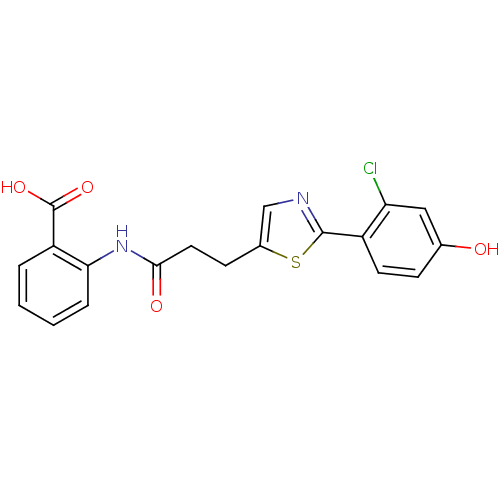
BDBM23525 2-{3-[2-(2-chloro-4-hydroxyphenyl)-1,3-thiazol-5-yl]propanamido}benzoic acid::Biaryl Anthranilide Analogue, 2g
SMILES OC(=O)c1ccccc1NC(=O)CCc1cnc(s1)-c1ccc(O)cc1Cl
InChI Key InChIKey=OLXPXHFQDBIPDV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 23525
Found 3 hits for monomerid = 23525
Affinity DataIC50: 1nM EC50: 6nMpH: 7.4 T: 2°CAssay Description:Membranes were incubated in binding buffer with [5, 6-3H]-niacin in the presence of test compound. After 4 hours at room temperature, reactions were ...More data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Agonist activity at human recombinant GPR109A receptor expressed in CHO-K1 cells after 60 mins by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]nicotinic acid from human GPR109A receptorMore data for this Ligand-Target Pair