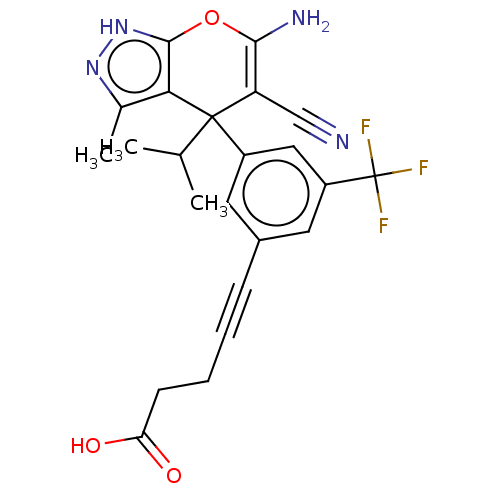
BDBM285658 US10077273, Compound 4::US10584132, Compound 4::US10934305, Compound 4
SMILES CC(C)C1(c2c(C)n[nH]c2OC(N)=C1C#N)c1cc(cc(c1)C(F)(F)F)C#CCCC(O)=O
InChI Key
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 285658
Found 3 hits for monomerid = 285658
TargetSerine hydroxymethyltransferase, mitochondrial(Homo sapiens (Human))
The Trustees of Princeton University
US Patent
The Trustees of Princeton University
US Patent
Affinity DataIC50: 0.170nMpH: 7.4Assay Description:For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate...More data for this Ligand-Target Pair
TargetSerine hydroxymethyltransferase, cytosolic(Homo sapiens (Human))
The Trustees of Princeton University
US Patent
The Trustees of Princeton University
US Patent
Affinity DataIC50: 0.170nMAssay Description:For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate...More data for this Ligand-Target Pair
TargetSerine hydroxymethyltransferase, mitochondrial(Homo sapiens (Human))
The Trustees of Princeton University
US Patent
The Trustees of Princeton University
US Patent
Affinity DataIC50: 0.170nMAssay Description:Inhibition of human SHMT by compounds in this disclosure is evaluated, for example, in their racemic forms in an in vitro assay. For example, Compoun...More data for this Ligand-Target Pair