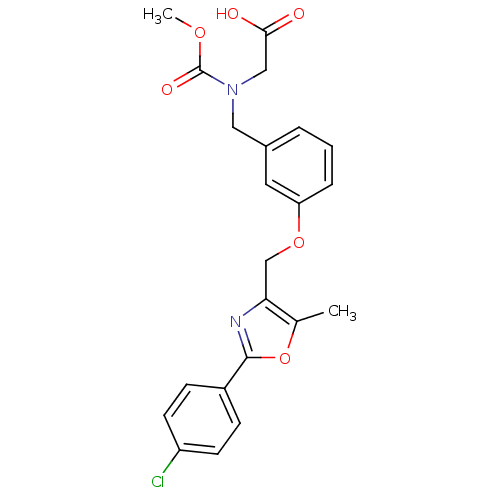
BDBM28800 2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-yl]methoxy}phenyl)methyl](methoxycarbonyl)amino}acetic acid::BMS-687453
SMILES COC(=O)N(CC(O)=O)Cc1cccc(OCc2nc(oc2C)-c2ccc(Cl)cc2)c1
InChI Key InChIKey=UJIBXDMNCMEJAY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 28800
Found 10 hits for monomerid = 28800
Affinity DataIC50: 260nM EC50: 10nMpH: 8.0 T: 2°CAssay Description:For hPPAR alpha, percentage inhibition was calculated relative to unlabeled GW2331, which was used as the active site-specific competitive binder. Fl...More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of human CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 260nMAssay Description:Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb R & D
Curated by ChEMBL
Bristol-Myers Squibb R & D
Curated by ChEMBL
Affinity DataIC50: >1.50E+4nMAssay Description:Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of human CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Bristol-Myers Squibb R & D
Curated by ChEMBL
Bristol-Myers Squibb R & D
Curated by ChEMBL
Affinity DataIC50: >1.50E+4nM EC50: 4.10E+3nMpH: 8.0 T: 2°CAssay Description:For PPARgamma, the percentage inhibition was calculated relative to rosiglitazone, which was used as the active site-specific competitive binder. Flu...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)