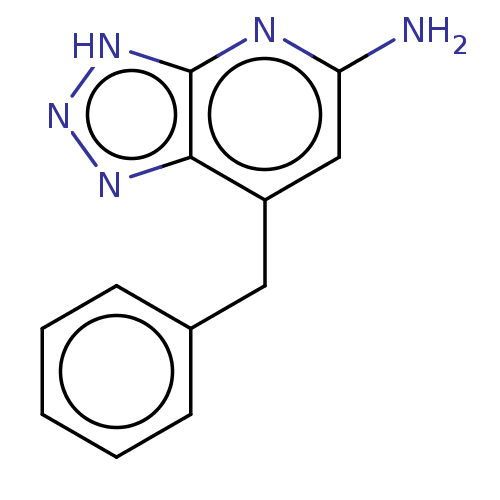
BDBM357629 7-benzyl-3H-[1,2,3]triazolo[4,5-b]pyridin-5-amine::US10214527, Example 1
SMILES Nc1cc(Cc2ccccc2)c2nn[nH]c2n1
InChI Key InChIKey=SGFFXIGIHATQNC-UHFFFAOYSA-N
Data 14 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 357629
Found 14 hits for monomerid = 357629
Affinity DataIC50: 55nMAssay Description:MPO chlorination activity was measured in 100 mM KPi (pH 7.4) by utilizing the non-fluorescent reagent Aminophenyl fluorescein (APF, Invitrogen catal...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:MPO peroxidation activity was measured in 100 mM KPi (pH 7.4) by utilizing the non-fluorescent reagent Amplex Red (Invitrogen catalog # A12222) which...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+4nMAssay Description:Inhibition of PMA-induced MPO in human Neutrophil incubated for 3 mins by luminometryMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of recombinant human MPO incubated for 10 mins in presence of 240 mM NaCl and 10 uM H2O2 by aminophenyl fluorescein based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:Inhibition of recombinant human MPO incubated for 10 mins by amplex red dye based assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 9.50E+3nMAssay Description:Inhibition of PMA-induced MPO in mouse Neutrophil incubated for 3 mins by luminometryMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of human EPX bromination activity using tyrosine as substrate by measuring 3-bromo tyrosine formation incubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of TPO (unknown origin) using 3-iodo tyrosine as substrate incubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2B6 (unknown origin)More data for this Ligand-Target Pair