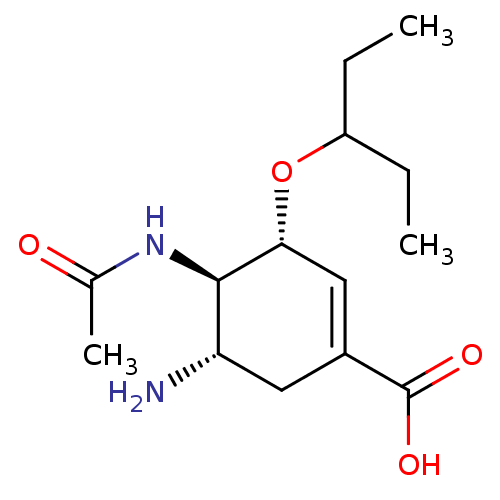
BDBM4994 (3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylic acid::CHEMBL674::GS4071::Oseltamivir carboxylate
SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O
InChI Key InChIKey=NENPYTRHICXVCS-YNEHKIRRSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 4994
Found 6 hits for monomerid = 4994
Affinity DataIC50: >1.00E+7nMAssay Description:Inhibition of human NEU1 expressed in HEK293 cells by fluorometric high-performance liquid chromatography using 4MU-NeuAc substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >6.00E+6nMAssay Description:Inhibition of human NEU2 expressed in HEK293 cells by fluorometric high-performance liquid chromatography using 4MU-NeuAc substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+7nMAssay Description:Inhibition of human NEU3 expressed in HEK293 cells by fluorometric high-performance liquid chromatography using 4MU-NeuAc substrateMore data for this Ligand-Target Pair
TargetNeuraminidase(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...)
Miyagi Cancer Center Research Institute
Curated by ChEMBL
Miyagi Cancer Center Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.10nMAssay Description:Inhibition of human Influenza A virus A/PR/8/34(H1N1) neuraminidase by fluorometric method using 4MU-NeuAc substrateMore data for this Ligand-Target Pair
TargetNeuraminidase(Influenza A virus (strain A/Aichi/2/1968 H3N2))
Miyagi Cancer Center Research Institute
Curated by ChEMBL
Miyagi Cancer Center Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human influenza A virus A/Aichi/2/1968(H3N2) neuraminidase by fluorometric method using 4MU-NeuAc substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+7nMAssay Description:Inhibition of human NEU4 expressed in HEK293 cells by fluorometric high-performance liquid chromatography using 4MU-NeuAc substrateMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)