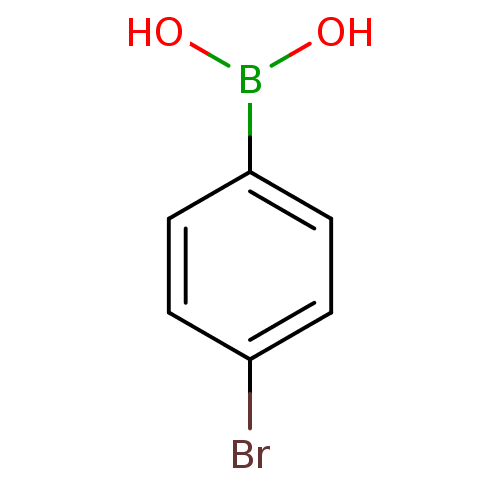
BDBM50067892 4-Bromophenyl-boronic acid::4-bromo phenyl boronic acid::4-bromophenyl boronic acid::4-bromophenylboronic acid::Boronic acid derivative::CHEMBL20866
SMILES OB(O)c1ccc(Br)cc1
InChI Key InChIKey=QBLFZIBJXUQVRF-UHFFFAOYSA-N
Data 10 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50067892
Found 10 hits for monomerid = 50067892
Affinity DataKi: 3.60E+3nMAssay Description:Inhibitory activity against E. coli AmpC beta-lactamase.More data for this Ligand-Target Pair
Affinity DataKi: 7.00E+3nMAssay Description:Inhibition of human recombinant cytosolic carbonic anhydrase 2 preincubated for 15 mins by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Competitive inhibition of Subtilisin BL wild type enzyme from B. lentusMore data for this Ligand-Target Pair
Affinity DataKi: 1.17E+4nMAssay Description:Inhibition of human recombinant cytosolic carbonic anhydrase 1 preincubated for 15 mins by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.59E+4nMAssay Description:Inhibition of Candida albicans recombinant Carbonic anhydrase preincubated for 15 mins by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+4nMAssay Description:Competitive inhibition of Subtilisin BL M222C-mutated enzyme from B. lentusMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+4nMAssay Description:Competitive inhibition of Subtilisin BL wild type enzyme from B. lentusMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+4nMAssay Description:Competitive inhibition of Subtilisin Carlsberg enzyme from B. lentusMore data for this Ligand-Target Pair
Affinity DataKi: 2.80E+4nMAssay Description:Inhibitory constant was determined against class A RTEM-1 Beta-lactamase from Escherichia coliMore data for this Ligand-Target Pair
Affinity DataKi: 2.20E+5nM ΔG°: -5.19kcal/molepH: 7.0 T: 2°CAssay Description:The inhibition studies of soybean urease were initiated with boric acid and boronic acids (butylboronic acid, 4-bromophenylboronic acid, and phenylbo...More data for this Ligand-Target Pair