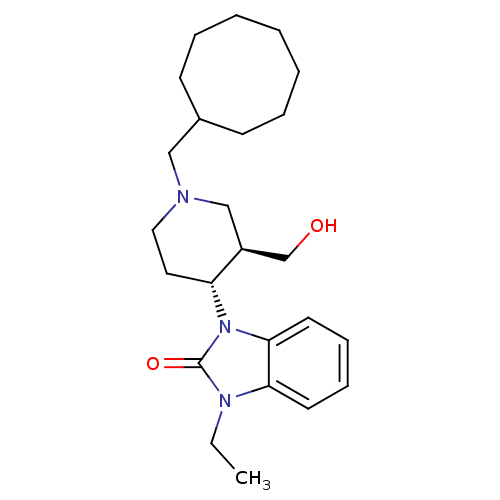
BDBM50083230 1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-piperidin-4-yl)-3-ethyl-1,3-dihydro-benzoimidazol-2-one::1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidinyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one::1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one::CHEMBL357076::J-113397
SMILES CCn1c2ccccc2n([C@@H]2CCN(CC3CCCCCCC3)C[C@H]2CO)c1=O
InChI Key InChIKey=MBGVUMXBUGIIBQ-LEWJYISDSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50083230
Found 2 hits for monomerid = 50083230
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity on nociceptin-induced maximal [35S]GTP-gamma-S binding to ORL1 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Agonistic activity of the compound against nociceptin produced GTPgammaS binding to Opioid receptor like 1 expressed in CHO cellsMore data for this Ligand-Target Pair