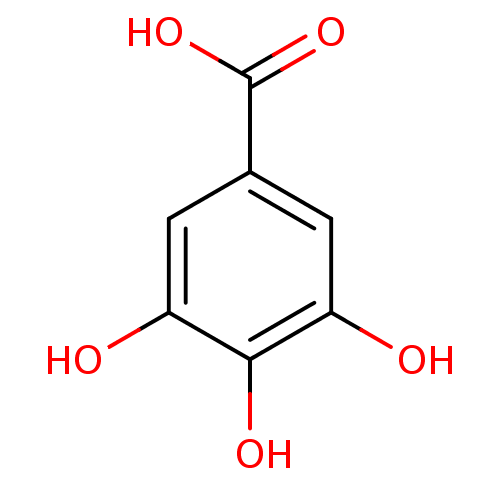
BDBM50085536 3,4,5-Trihydroxybenzoate, X::3,4,5-trihydroxybenzoic acid::CHEMBL288114::Gallic Acid, F::gallic acid
SMILES OC(=O)c1cc(O)c(O)c(O)c1
InChI Key InChIKey=LNTHITQWFMADLM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 16 hits for monomerid = 50085536
Found 16 hits for monomerid = 50085536
Affinity DataKi: 2.25E+3nMAssay Description:Inhibition of human CA2 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.20E+3nMAssay Description:Inhibition of human CA1 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.08E+3nMAssay Description:Inhibition of human CA5A by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.13E+3nMAssay Description:Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.07E+3nMAssay Description:Inhibition of human CA7 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.13E+3nMAssay Description:Inhibition of human CA6 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.99E+3nMAssay Description:Inhibition of human CA9 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.03E+3nMAssay Description:Inhibition of human CA14 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.49E+3nMAssay Description:Inhibition of human CA3 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.78E+3nMAssay Description:Inhibition of human CA12 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 9.80E+3nMAssay Description:Inhibition of human CA4 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 9.86E+3nMAssay Description:Inhibition of mouse CA13 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 9.97E+3nMAssay Description:Inhibition of human CA5B by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+4nMAssay Description:Inhibition assay using procollagen-prolin, 2-oxoglutarate 4-dioxygenase.More data for this Ligand-Target Pair
Affinity DataKi: 7.58E+5nM ΔG°: -4.25kcal/molepH: 7.4 T: 2°CAssay Description:Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion using s...More data for this Ligand-Target Pair
Affinity DataKi: 1.05E+6nM ΔG°: -4.06kcal/molepH: 7.4 T: 2°CAssay Description:Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion using s...More data for this Ligand-Target Pair