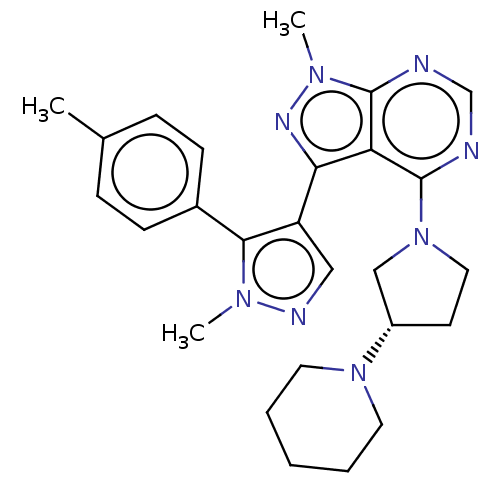
BDBM50088503 CHEMBL3527048
SMILES Cc1ccc(cc1)-c1c(cnn1C)-c1nn(C)c2ncnc(N3CC[C@@H](C3)N3CCCCC3)c12
InChI Key InChIKey=WDWIMDKOXZZYHH-FQEVSTJZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 50088503
Found 15 hits for monomerid = 50088503
Affinity DataKi: 120nMAssay Description:Inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A4 harboring human P450 oxidoreductase and b5 assessed as decrease in enzyme...More data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A4 in presence of human P450 oxidoreductase and b5 assessed as decreas...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A5 in presence of human P450 oxidoreductase and b5 assessed as decreas...More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A5 harboring human P450 oxidoreductase and b5 assessed as decrease in enzyme...More data for this Ligand-Target Pair
Affinity DataIC50: 7.70E+4nMAssay Description:Reversible inhibition of human CYP2C19 S-mephenytoin 4'-hydroxylase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 7.10E+4nMAssay Description:Reversible inhibition of human CYP2D6 dextromethorphan O-demethylase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Reversible inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A4 harboring human P450 oxidoreductase and b5More data for this Ligand-Target Pair
Affinity DataIC50: 690nMAssay Description:Reversible inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A4 in presence of human P450 oxidoreductase and b5More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Reversible inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A5 harboring human P450 oxidoreductase and b5More data for this Ligand-Target Pair
Affinity DataIC50: 7.10E+4nMAssay Description:Reversible inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A5 in presence of human P450 oxidoreductase and b5More data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMAssay Description:Reversible inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A7 harboring human P450 oxidoreductase and b5More data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+4nMAssay Description:Reversible inhibition of human CYP2C8 paclitaxel 6alpha-hydroxylase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 8.80E+4nMAssay Description:Reversible inhibition of human CYP2B6 bupropion hydroxylase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+4nMAssay Description:Reversible inhibition of human CYP1A2 phenacetin O-deethylase activityMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Reversible inhibition of human CYP2C9 diclofenac 4'-hydroxylase activityMore data for this Ligand-Target Pair