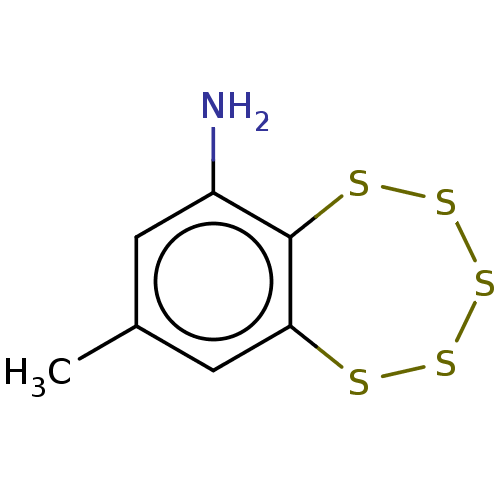
BDBM50103444 CHEMBL3398244
SMILES Cl.Cc1cc(N)c2SSSSSc2c1
InChI Key InChIKey=CGAFPYIEPKSPSH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50103444
Found 3 hits for monomerid = 50103444
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 116nMAssay Description:Inhibition of STEP (unknown origin) in absence of GSH by p-nitrophenyl phosphate hydrolysis assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of STEP (unknown origin) pre-incubated for 10 mins before addition of p-nitrophenyl phosphate substrate in absence of GSHMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of STEP (unknown origin) pre-incubated for 10 mins before addition of p-nitrophenyl phosphate substrate in presence of 1 mM GSHMore data for this Ligand-Target Pair