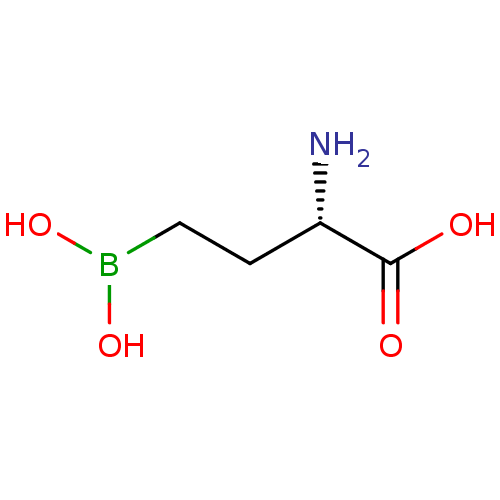
BDBM50104413 CHEMBL87796::Glutamyl-gamma-boronate analogue
SMILES N[C@@H](CCB(O)O)C(O)=O
InChI Key InChIKey=KSYFGBKMRXVJSG-VKHMYHEASA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50104413
Found 4 hits for monomerid = 50104413
TargetGlutathione hydrolase 1 proenzyme(Homo sapiens (Human))
State University Of New York
Curated by ChEMBL
State University Of New York
Curated by ChEMBL
Affinity DataKi: 5.40nMAssay Description:Inhibition of human GGT1 transpeptidation in Pichia pastoris using L-GpNA as substrate in presence of Gly-GlyMore data for this Ligand-Target Pair
TargetGlutathione hydrolase 1 proenzyme(Homo sapiens (Human))
State University Of New York
Curated by ChEMBL
State University Of New York
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:Inhibition of human GGT1 expressed in Pichia pastoris assessed as inhibition of GSH hydrolysis measured by L-glutamate release assayMore data for this Ligand-Target Pair
TargetGlutamyl-tRNA(Gln) amidotransferase subunit C(Streptococcus pyogenes serotype M1)
Dupont Pharmaceuticals
Curated by ChEMBL
Dupont Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:In vitro inhibitory concentration against transferase activity of bacterial Glu-tRNA-Gln amidotransferase (Glu-AdT)More data for this Ligand-Target Pair
TargetGlutamyl-tRNA(Gln) amidotransferase subunit C(Streptococcus pyogenes serotype M1)
Dupont Pharmaceuticals
Curated by ChEMBL
Dupont Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:In vitro inhibitory concentration against Glutaminase activity of bacterial Glu-tRNA-Gln amidotransferase (Glu-AdT)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)