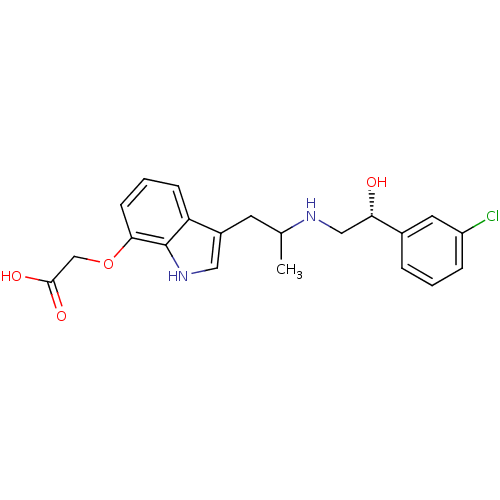
BDBM50126078 (3-{2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-1H-indol-7-yloxy)-acetic acid::CHEMBL282190
SMILES CC(Cc1c[nH]c2c(OCC(O)=O)cccc12)NC[C@H](O)c1cccc(Cl)c1
InChI Key InChIKey=FHEYFIGWYQJVDR-UWBLVGDVSA-N
Data 4 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50126078
Found 4 hits for monomerid = 50126078
Affinity DataEC50: 0.110nMAssay Description:Agonism of recombinant human beta-3 adrenergic receptor assayed by measuring cAMP accumulation in CHO cells expressing beta3-ARMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
National Institute Of Pharmaceutical Education And Research
Curated by ChEMBL
National Institute Of Pharmaceutical Education And Research
Curated by ChEMBL
Affinity DataEC50: 13nMAssay Description:Agonist activity at Homo sapiens (human) beta2 adrenoreceptorMore data for this Ligand-Target Pair
TargetBeta-1 adrenergic receptor(Homo sapiens (Human))
National Institute Of Pharmaceutical Education And Research
Curated by ChEMBL
National Institute Of Pharmaceutical Education And Research
Curated by ChEMBL
Affinity DataEC50: 6.40nMAssay Description:Agonist activity at Homo sapiens (human) beta1 adrenoreceptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0620nMAssay Description:Agonist activity at Homo sapiens (human) beta3 adrenoreceptorMore data for this Ligand-Target Pair