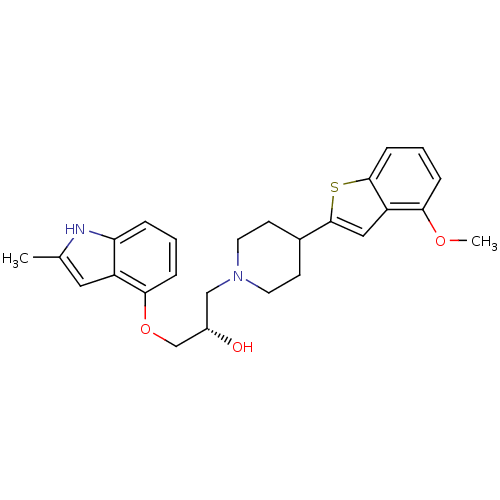
BDBM50128370 (S)-1-(4-(4-methoxybenzo[b]thiophen-2-yl)piperidin-1-yl)-3-(2-methyl-1H-indol-4-yloxy)propan-2-ol::(S)-1-[4-(4-Methoxy-benzo[b]thiophen-2-yl)-piperidin-1-yl]-3-(2-methyl-1H-indol-4-yloxy)-propan-2-ol::CHEMBL300340
SMILES COc1cccc2sc(cc12)C1CCN(C[C@H](O)COc2cccc3[nH]c(C)cc23)CC1
InChI Key InChIKey=SYWVBRYJGFTBHV-IBGZPJMESA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50128370
Found 4 hits for monomerid = 50128370
Affinity DataKi: 4.80nMAssay Description:Binding affinity was determined towards 5-hydroxytryptamine 1A receptor receptor using [3H]-8-OH-DPAT as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 4.83nMAssay Description:Displacement of [3H]8-OH-DPAT from 5HT1A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 51nMAssay Description:Binding affinity towards serotonin [5-HT] reuptake site labeled with [3H]-paroxetine as radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 51.2nMAssay Description:Displacement of [3H]paroxetine from 5HT reuptake siteMore data for this Ligand-Target Pair