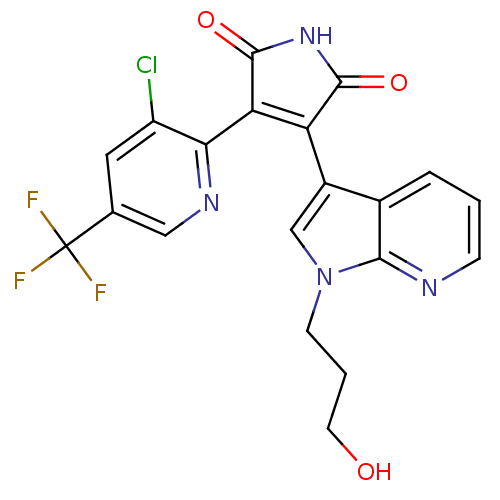
BDBM50147463 3-(3-Chloro-5-trifluoromethyl-pyridin-2-yl)-4-[1-(3-hydroxy-propyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]-pyrrole-2,5-dione::CHEMBL111620
SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ncc(cc2Cl)C(F)(F)F)c2cccnc12
InChI Key InChIKey=WFTBLYUVDBPJAV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50147463
Found 7 hits for monomerid = 50147463
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrateMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of Cyclin-dependent kinase 2-cyclin AMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 5.80E+3nMAssay Description:Inhibition of Cyclin-dependent kinase 1-cyclin BMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Inhibition of human protein kinase C etaMore data for this Ligand-Target Pair
TargetRibosomal protein S6 kinase alpha-5(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of MSK-1 kinaseMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Inhibition of GSK-3beta (unknown origin)More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataEC50: 620nMAssay Description:Effective concentration of compound against glycogen synthase kinase-3 in HEK293 cellsMore data for this Ligand-Target Pair