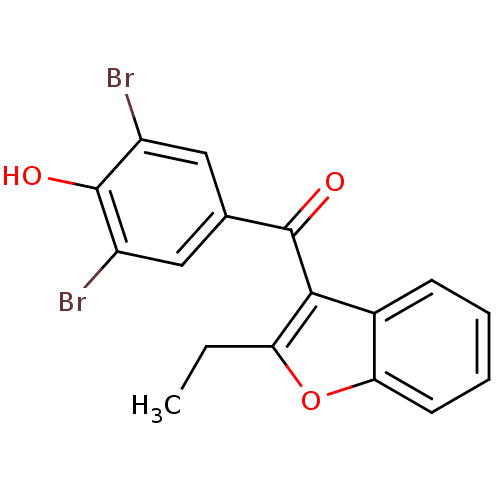
BDBM50158460 (3,5-dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran-3-yl)methanone::2-ethyl-3-(3,5-dibrom-4-hydroxybenzoyl)benzofuran::3,5-dibromo-4-hydroxyphenyl-2-ethyl-3-benzofuranyl ketone::Benzbromarone::CHEMBL388590::US9725430, Compound 1::US9856239, benzbromarone::US9962362, Compound 1::Uroleap (TN)::cid_2333
SMILES CCc1oc2ccccc2c1C(=O)c1cc(Br)c(O)c(Br)c1
InChI Key InChIKey=WHQCHUCQKNIQEC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50158460
Found 3 hits for monomerid = 50158460
TargetEyes absent homolog 3 [223-510](Homo sapiens (Human))
Cincinnati Childrens Hospital Medical Center
US Patent
Cincinnati Childrens Hospital Medical Center
US Patent
Affinity DataIC50: 1.10E+4nMAssay Description:An inhibitory assay was conducted using the previously described p-nitrophenylphosphate assay (Rayapureddi, J. P. et al. Nature 426, 295-298 (2003))....More data for this Ligand-Target Pair
TargetEyes absent homolog 3(Homo sapiens (Human))
Cincinnati Childrens Hospital Medical Center
US Patent
Cincinnati Childrens Hospital Medical Center
US Patent
Affinity DataIC50: 8.30E+3nMAssay Description:An inhibitory assay was conducted using the previously described p-nitrophenylphosphate assay (Rayapureddi, J. P. et al. Nature 426, 295-298 (2003))....More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Cincinnati Childrens Hospital Medical Center
US Patent
Cincinnati Childrens Hospital Medical Center
US Patent
Affinity DataIC50: 5.38E+4nMAssay Description:An inhibitory assay was conducted using the previously described p-nitrophenylphosphate assay (Rayapureddi, J. P. et al. Nature 426, 295-298 (2003))....More data for this Ligand-Target Pair