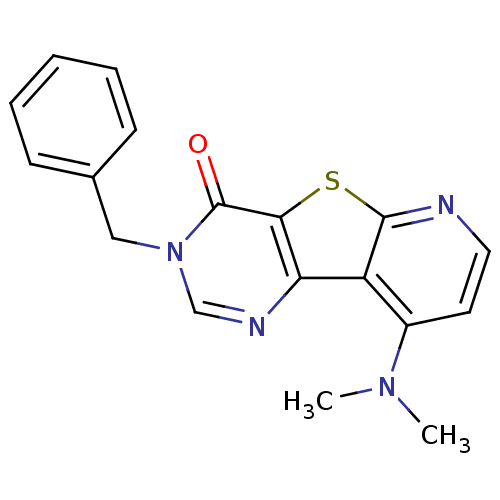
BDBM50177064 3-Benzyl-9-dimethylamino-3H-pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-one::9-dimethylamino-3-benzyl-3H-5-thia-1,3,6-triazafluoren-4-one::CHEMBL388087::cid_657896
SMILES CN(C)c1ccnc2sc3c(ncn(Cc4ccccc4)c3=O)c12
InChI Key InChIKey=AVJJIBOWEKYOER-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50177064
Found 9 hits for monomerid = 50177064
Affinity DataKi: 87nMAssay Description:Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranesMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Rattus norvegicus (Rat))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]MPEP from rat cortex mGluR5More data for this Ligand-Target Pair
TargetSphingosine 1-phosphate receptor 1(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataEC50: 2.47E+3nMAssay Description:Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute, TSRI Assay ...More data for this Ligand-Target Pair
TargetUbiquitin-conjugating enzyme E2 N(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: >2.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
Affinity DataIC50: 281nMAssay Description:Inhibition of rat mGluR1More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Antagonist activity at human mGluR5 expressed in 1321N1 cells assessed as effect on L-glutamate-induced calcium mobilizationMore data for this Ligand-Target Pair
TargetSphingosine 1-phosphate receptor 1(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataEC50: >9.51E+4nMAssay Description:Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute, TSRI Assay ...More data for this Ligand-Target Pair
TargetSphingosine 1-phosphate receptor 1(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataEC50: 3.37E+3nMAssay Description:Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute, TSRI Assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 281nMAssay Description:Antagonist activity at human mGluR1 expressed in 1321N1 cells assessed as effect on L-glutamate-induced calcium mobilizationMore data for this Ligand-Target Pair